Unit for Experimental Psychiatry, The Institute of Pennsylvania Hospital,
and Department of Psychiatry, University of Pennsylvania School of Medicine, Philadelphia, Pennsylvania 19139-2798, USA*
Prolonged work scenarios with demands for sustained performance are increasingly common. Because sleep loss inevitably compromises functioning in such situations, napping has been proposed as a countermeasure. The optimal timing of the nap relative to its benefits for performance and mood is not known, however. To address this issue, 41 healthy adults were permitted a two-hour nap at one of five times during a 56-hour period of intermittent work, with no other sleep. Naps were placed 12 hours apart, near the circadian peak (P) or trough (T), and were preceded by 6(P), 18(T), 30(P), 42(T), or 54(P) hours of wakefulness. Work test bouts occurred every few hours and consisted of a variety of psychomotor and cognitive tasks as well as mood scales completed at the beginning, middle and end of each bout. A total of eight performance and 24 mood parameters were derived from the bouts and compared between groups at all test points prior to and following the naps. An estimate of the extent to which each nap condition differed from the control (P54) condition was derived by totalling the proportion of test points that yielded statistically significant results relative to the total number of tests conducted both before and after naps.
Although all performance and most mood parameters displayed a circadian-modulated deterioration as the protocol progressed, a nap appeared to attentuate the extent of this change in all performance parameters but not in mood parameters. Overall, the timing of the nap across days and within the circadian cycle was irrelevant to its effect on performance, suggesting that it diminished the intrusion of sleepiness into behavioural functioning, even though subjects were phenomenally unaware of this benefit.
Keywords: napping, sleep, fatigue, performance, mood.
The requirement to remain awake and function for periods of time beyond the typical daily wake-sleep ratio of 2 : 1 (16: 8 h) is an increasingly common aspect of some work settings. Examples include participation in sustained military operations, preparatory actions for complex and costly ventures (e.g. launching a manned space flight), and many types of crisis or catastrophe management (e.g. forest fires or nuclear power plant alerts). In most of these situations the prolonged work or readiness schedule not only results in a loss of sleep, but co
139
140 D. F. Dinges et al.
exists with a continuous or intermittent demand for very high levels of accurate performance and sustained motivation and morale. The demand for optimal performance is itself a result of the consequences that a human error can have for health and safety in many of these scenarios.
Given the need for optimal functioning in such situations, it is hardly surprising that there has been considerable interest in discovering effective countermeasures to the decline in performance and mood that result from prolonged wakefulness. Although notions of mental and physical 'toughness' as well as prior experience with sleep loss continue to be popular countermeasure concepts, there is not much evidence that these factors prevent sleepiness and its behavioural consequences, although they may motivate the person to try harder to overcome these effects. In fact, there appears to be no substitute for sleep itself. If this is the case, then the question of countermeasures focuses on obtaining sleep in small amounts, whenever there is an opportunity during a quasi-continuous work scenario. A short period of sleep obtained ad lib is an apt definition of a 'nap' and hence there has been considerable interest in the benefits of naps, in lieu of longer sleep opportunities, for persons undergoing prolonged work situations.
Many napping studies carried out in the past 15 years show that, as one might expect, a single nap from one to four hours duration taken during a 24-hour period of otherwise sustained wakefulness, with or without work demands, can significantly attenuate the behavioural effects of sleepiness relative to no sleep at all (Angiboust and Gouars 1972, Gillberg 1984, Haslam 1981, Lumley et al. 1986, Nicholson et al. 1985, Opstad et al. 1978, Thorne et al. 1983). Generally, the longer the nap, the greater the benefits (Haslam 1981, Lumley et al. 1986, Morgan 1974, Opstad et al. 1978).
What remains unanswered in this work is the precise nature of the benefits on mood and performance derived from naps, as well as the likelihood of such benefits as a function of the temporal placement of naps within the circadian cycle and across successive days of sleep loss. A practical question of interest to persons planning such scenarios is: If you can only sleep for an hour or two during a two-to-three-day period of sustained activity, when should you take that nap and to what extent would mood and performance change for the better as a function of having taken it?
There is some evidence that a nap taken near the circadian nadir in body temperature may be less beneficial than one taken at a higher phase of the circadian cycle (cf. Naitoh 1981, Naitoh et al. 1982). Similarly, most studies have focused on the effects of naps following a day or two without sleep, despite the suggestion that napping early in the protocol, prior to sleep loss effects -- what Orne (Evans and Orne 1976) called 'prophylactic' napping -- may yield benefits (cf. Angiboust and Gouars 1972, Gillberg 1984). Recently, the authors reported the only study to date that varied both the circadian placement of the nap and the amount of prior wakefulness throughout a 56-hour protocol (Dinges et al. 1987). The effects of varying the temporal placement of the nap on simple visual reaction time (VRT), Stanford Sleepiness Scale (SSS) ratings, and sublingual temperature rhythms suggested that, regardless of the circadian phase in which it occurred, the nap benefited VRT performance but not SSS mood ratings. The positive effects on VRT were especially evident for naps taken early in the protocol, despite the fact that these early naps were comprised of lighter sleep (Dinges 1986).
This report provides further data that delineates the varied nature of nap effects on mood and performance during sustained wakefulness protocols, and extends the assessment of these effects to eight different performance parameters and 24 aspects of mood reports, to evaluate the full range of nap benefits relative to its temporal placement and to determine whether the benefits are confined to performance.
141 The benefits of napping
Method
The study design, subject characteristics, and experimental procedures, described in detail elsewhere (Dinges 1986, Dinges et al. 1987), are briefly reviewed here, along with the types of measures employed and the nature of the data analyses.
Design
A between-groups design was used with five conditions that differed only in temporal placement of a two-hour nap opportunity during a 56-hour period of otherwise sustained wakefulness with intermittent performance demands in a controlled laboratory environment. For a given condition, a two-hour nap could occur near either the circadian peak (P = circa 15.00 h) or near the trough (T = circa 03.00 h) in the activity cycle. The P and T designates were based on clock time, but post hoc analyses revealed that these designates were reasonable because P naps always occurred a few hours prior to the peak of subjects' circadian temperature cycles while T naps occurred a few hours before subjects' circadian nadir in temperature.
The five conditions differed in the amount of sustained wakefulness prior to the nap, which was measured from the time subjects awoke from nocturnal sleep on the morning of the first day of the 56-hour protocol. The earliest nap condition was scheduled at 15.00 h of the first day, about six hours after the morning awakening, and hence was designated the P6 condition. Twelve hours later, at 03.00 h and after 18 hours of wakefulness, a two-hour nap opportunity was offered in the T18 condition. The P30 condition provided a two-hour nap 12 hours after T18 (30 hours of prior wakefulness) on the afternoon of the second day. The fourth condition, T42, provided a nap opportunity 12 hours after P30, during the second night. P54 served as the control condition, providing a nap 12 hours after T42, on the afternoon of the third day, near the end of the protocol. Thus, all conditions allowed for no more than two hours of sleep and no less than 54 hours of wakefulness, and both circadian placement of the nap as well as wakefulness prior to the nap were varied systematically.
Subjects
Forty-two healthy young adults (27 males and 15 females) between the ages of 18 and 30 (mean age 20.4 years, SD 2.7 years) were randomly assigned to one of five conditions, with the requirement that conditions were to be equivalent in gender composition (the gender imbalance reflected the fact that fewer females than males volunteered for the study). While the four treatment conditions had eight subjects each, the control group (P54) had ten subjects; however, the data from the tenth P54 subject was eliminated before analyses upon discovery that he had ingested alcohol just prior to the sleep loss segment. Subjects were run through the trial in same-sex pairs, they received a token reimbursement of $150 for participating, and they were informed that they could withdraw from the study at any time without jeopardy. An extensive debriefing interview was conducted at the end of the experiment and follow-up contact was maintained with each participant. All subjects completed the study and encountered no problems after a night of recovery sleep.
Protocol
Two baseline sessions, each five hours in duration, took place during the two weeks prior to the 56-hour protocol. These sessions served to acquaint subjects with the study procedures and facilities, provide them practice sessions for the performance tasks and completion of the
142 D. F. Dinges et al.
self-report measures, and ensure they maintained their usual sleep-wake cycles during the study. The latter was also checked by having them complete a daily sleep diary during the two weeks prior to the protocol. Subjects reported to the laboratory around noon on the first day of the 56-hour protocol. They remained in the laboratory working under behavioural observation until about 18.00h two days later (on the third day).
Activities throughout the 56-hour protocol were arranged such that only the placement of naps differed among groups. Subjects were told that some time during the 56 hours a nap of from ten minutes to five hours duration would be permitted, but they did not know when it would occur. In fact, they were awakened two hours after being told to go to sleep. The naps took place on beds in dark, quiet bedrooms. Sleep was polygraphically recorded and scored by standardized criteria. The effects of temporal placement on nap sleep psychophysiology have been reported elsewhere (Dinges 1986) and will be discussed here only to the extent that it is relevant to the effects of the naps on other measures.
Throughout the 56-hour protocol subjects were monitored continuously by behavioural observation to ensure that they remained awake. Performance work bouts began on average every 2.4 hours throughout the 56-hour protocol and lasted approximately 45-60 minutes each time. Between work bouts subjects were free to read, play games, and engage in conversation, but not to sleep. Meals took place four times a day, at times appropriate for breakfast, lunch, supper, and a mid-nocturnal snack; the foods offered were of the type normally consumed at these times. Although the environment was free of time cues (e.g. windows were occluded and there were no clocks, televisions, radios, or telephons, etc.) to prevent knowledge of time from influencing performance effort or mood reports of subjects, the timing of the meals provided some temporal cues. This was considered acceptable, since healthy adults can readily predict during a three-day period such broad variations in time without cues, but they cannot predict the precise hour of the day, and the meals were timed so that the hour was unclear.
Debriefing interviews revealed that most subjects felt that the intent of the study was to measure response to sleep loss or temporal isolation or both. The between-subject design, together with staff discretion, prevented subjects from knowing that other nap conditions were also being tested. Consequently, the debriefing interviews determined that only two subjects guessed the intent of the experiment, which suggests the results reported here were not due to a differential motivation or response to demand characteristics (Orne 1962).
Performance
The performance work bouts included a variety of short-duration (1-20 min) cognitive and psychomotor tasks that are frequently used in studies of sleep loss, shift work, and human chronobiology. In addition, some of the tasks, such as the VRT were subdivided into optimal performance capacity (fastest 10% of responses), median performance capacity (median 10% of responses), and performance lapses (slowest 10% of responses) as described in the authors' earlier report (Dinges et al. 1987). These were administered in a predetermined random order by an experimenter who was present with the subject throughout the test. Subjects were continually urged to do their very best on each task. This instruction, the brevity of the tasks, their random placement in the bout, and the presence of the experimenter were intended to keep subjects' motivation to perform at the highest possible level while in the work room.
Although some cognitive tasks were administered in alternate test bouts, a simple, tenminute portable, unprepared VRT task developed by Wilkinson (Wilkinson and Houghton 1982) was administered virtually every bout. This task was developed for
143 The benefits of napping
assessment of alertness in field situations and has been shown consistently to be sensitive to as little as one night's sleep loss (Glenville et al. 1978), to partial sleep loss (Herscovitch and Broughton 1981, Tilley and Wilkinson 1984), and to circadian variation in performance efficiency (Glenville and Wilkinson 1979, Tilley et al. 1982). The manner in which the VRT data obtained from it was analysed involved a special microcomputer software system the authors developed (Dinges and Powell 1985) to evaluate three aspects of performance capacity: optimum responding (fastest 10%), response slowing (median %), and response lapsing (slowest 10%).
The most frequently administered cognitive tasks in the work bouts included the threeminute Descending Subtraction Task (DST) (Evans and Orne 1975), developed as a measure that places a heavy load on short-term memory (Dinges et al. 1985); the Digit-Span (D-S) task from the Wechsler Scale; and the Memory and Search Task (MAST) used to assess different performance rhythms (Folkard et al. 1976). DST performance was measured as the number of correct subtractions per three-minute trial; D-S performance was measured as both total score and score to first error; MAST performance was measured as the times to complete the two-letter and six-letter target columns. For each of these cognitive tasks multiple sets of stimulus sequences were used to prevent subjects from becoming familiar with any one sequence. The five dependent variables derived from performance on these tasks together with the three VRT response variables resulted in a total of eight performance measures. Other performance tasks, such as auditory reaction time (Lisper and Kjellberg 1972) and a dual-probe memory recognition task (Dinges and Whitehouse 1985), were administered in work bouts to provide some diversity from bout to bout, but they were not included here because they yielded redundant information or were performed too infrequently for analysis of temporal pattern over the 56-hour protocol.
Mood
A number of subjective self-report scales were completed both at the beginning (pre-test) and end (post-test) of each performance work bout, while sublingual temperature was being recorded, because work itself appears to alter the temporal pattern of mood at certain circadian phases (cf. Dinges et al. 1984, Angus and Heslegrave 1985). Hence, each work bout began and ended with the SSS (Hoddes et al. 1973), the Profile of Mood States (POMS) (McNair et al. 1971), and six-point Fatigue and Tension scales (Tepas 1980). The six subscales scores from the POMS together with the SSS and Fatigue and Tension ratings resulted in a total of nine pre-test and nine post-test mood scores. Two other self-report scales were completed in the middle (mid-test) of each performance bout; these were the Activation-Deactivation Adjective Checklist (AD-ACL) (Thayer 1967, 1986) and the NPRU (Johnson and Naitoh 1974). The AD-ACL yields four mood subscales and the NPRU provides a positive and negative mood score, for a total of six mid-test mood scores. Thus, a grand total of 24 mood variables (nine pre-test, nine post-test, and six mid-test) were examined across the 56-hour protocol. Analog sleepiness and fatigue scales were also assessed in work bouts, but these are not discussed here because they are redundant.
Data analyses
Each of the eight performance variables was measured as a percentage of the final baseline trial performance (the end of the practice sessions prior to the experiment) with each subject, upon determination that the five groups did not differ significantly from each other at the
144 D. F. Dinges et al.
end of baseline. This was done to provide a relative metric across performance tasks in terms of percentage gain or loss on a task. The raw scores of mood variables were used instead of percentages, because of the problem of zero scores on some parameters. Nevertheless, when mood was analysed as percentage of baseline, eliminating all data points that required division by zero, the results were comparable to those presented below for raw scores.
For each of the 32 variables (eight performance and 24 mood) the temporal pattern across the 56 hour period was averaged within each of the five groups and plotted. The four nap treatment groups (P6, T18, P30, T42) were compared separately at each time point to the P54 control group. The comparisons for performance variables (percentages) were done nonparametrically (Mann-Whitney U-test), while mood variables were analysed parametrically (t-test and F-test for homogeneity of independent variances).
The strategy of comparing each nap group separately to the control group, at each time point before and after the nap, is presented here, but other approaches to the data, such as the use of ANOVAS to compare all groups at each time point were also conducted and produced results consistent with those provided by the individual comparisons. The approach presented here was considered preferable because by comparing each nap condition to the control condition the relative benefits of a given nap could be assessed with regard to the temporal placement of the nap. With many individual comparisons an estimate of a given nap benefit could be determined, taking into account the expectation of no differences prior to a nap and the total number of comparisons conducted. For example, if a given nap condition improved any measure, then the measure should be significantly different after each nap condition ('predicted') when compared to the P54 control group, but there should be no differences before each nap ('not predicted'). Moreover, the proportion of significant differences relative to the total number of comparisons conducted should be well beyound what chance would allow (in this case circa 5%). Thus, if a nap yielded no significant differences on a parameter (relative to the P54 group) at all time points prior to its occurrence, but yielded significant differences at all time points after its occurrence, then that would be 100% of all 'predicted' (P) differences and 0% of all 'not predicted' (NP) differences -- which would indicate the nap had a consistent and prolonged effect on a given parameter. On the other hand, if a nap resulted in 8% P differences and 3% NP differences, there would be no support for assuming it altered the variable in a meaningful way, because chance alone would allow for 5 % of the comparisons to be significant at p < 0.05. The final step in this pattern assessment of the results consists of subtracting the proportion of NP differences from the proportion of P differences to derive an estimate of a nap benefit on each of the 32 parameters.
Results
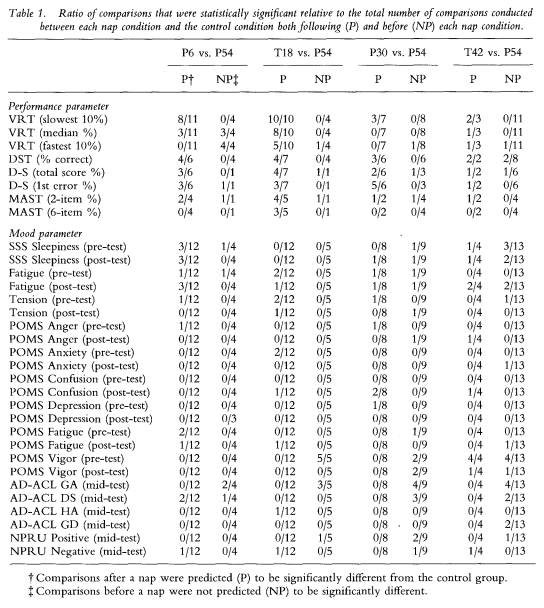
Table 1 displays the ratio of P and NP differences for all analyses conducted on each of the 32 variables between the P54 control condition and the four nap conditions (P6, T18, P30, T42). No differences were predicted for test bouts conducted before each nap and, as the table shows, few variables resulted in significant differences for tests conducted on trials prior to the naps (see the NP column).
To reveal how performance fared relative to mood, table 2 displays the column totals translated into percentages for the data in table 1. With the exception of the P6 group, the percentage of post-nap (predicted) differences for performance always exceeded the percentage of pre-nap (not predicted) differences, resulting in a total of 48% (87/182) of all P differences statistically significant at p < 0.05 or lower, relative to 13% (19/145) of all NP performance comparisons. In contrast, all P and NP percentages for mood were below 14%
145 The benefits of napping

for each group comparison, resulting in a total of 6% (50/864) of all post-nap mood differences statistically significant, relative to 8% (59/744) of all pre-nap mood comparisons. These percentages are just above the 5% one should expect to find significant by chance when conducting this number of comparisons on a data set and accepting significance at the 1 in 20 level.
Thus, the number of significant differences was considerably greater for performance variables, while mood variables produced results at the chance level. A close examination of table 1 reveals that this differential in the proportion of significant differences between performance and mood variables was the result of some performance measures being greatly affected by the naps, but virtually no mood parameter being systematically altered.
146 D. F. Dinges et al.
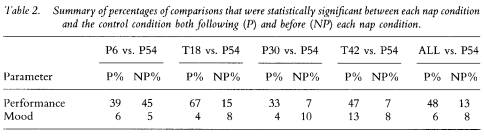
Although the effect of a nap on performance appeared to be greater for some measures (e.g. VRT slowest 10%) than for others (e.g. MAST 6-item %), all four nap conditions (P6, T18, P30, T42) appeared to show similar effects on a given performance parameter; the exceptions being variability in the ratio of significant outcomes within and between groups for the VRT median % and fastest 10%, which produced the spuriously high proportion of NP differences in the P6 group. To determine which parameters were most affected by a nap, regardless of when it was taken, the frequencies for the number of significant comparisons relative to the total number of comparisons were summed across nap groups, within each performance and mood variable. For example, in table 1 the P ratios for DST performance (% correct) are 4/6, 4/7, 3/6, and 2/2, which totals to 13/21 or 62%, The equivalent NP ratios for this variable were 0/4, 0/4, 0/6 and 2/8, resulting in a total of 2/22 or 9%. The difference between these total proportions (i.e. 62% minus 9% equals 53%) provides an estimate within this study of the extent to which a nap aided a given parameter, relative to no nap. That is, it is estimated that when corrected for pre-nap differences, a nap placed anywhere in the protocol enhanced DST performance approximately 53% of the time from the nap to the end of the protocol, relative to no sleep at all. Table 3 displays the estimates in rank order of the difference in P and NP proportions for the ten parameters most affected by a nap.
VRT performance in the lapse domain (slowest 10%) was most consistently affected by a nap (P ratios) without showing any pre-nap differences (NP ratios) among groups, and was followed by an aspect of each of the four performance tasks analysed. In fact, the seven highest difference scores in table 3 were derived from performance variables, leaving only one performance parameter (VRT fastest 10%) out of the top 10. Only three of the 24 mood variables appear in this list and they result in difference scores between 8% and 12%; the remaining 21 mood variables yielded difference scores below 7%, which is at the chance level.
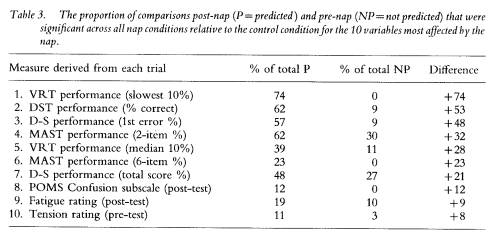
In order to determine the manner in which the differences observed as a function of a nap were related to circadian and sleep loss effects, plots of each parameter were visually examined. As expected, most mood variables and all performance variables showed a significant variation over time as a function of sleep loss; a circadian rhythm could also be seen within this variation. The positive effect of a nap on performance took the form of preventing or attentuating this circadian-modulated decline in performance. Figure 1 displays the plots for DST performance (% correct). Data from each of the four nap groups (P6, T18, P30, T42) are compared to the P54 control group data.
While the P54 group began to decline from 100% of baseline as sleep loss progressed (i.e. decreasing numbers of correct subtractions) each nap appeared to delay this decline. Although overall DST performance was not affected as negatively by sleep loss as was VRT performance in the lapse domain (cf. Dinges et al. 1987), the effect of a nap relative to the same performance time without a nap was identical across the two measures. A nap resulted in better performance.
147 The benefits of napping
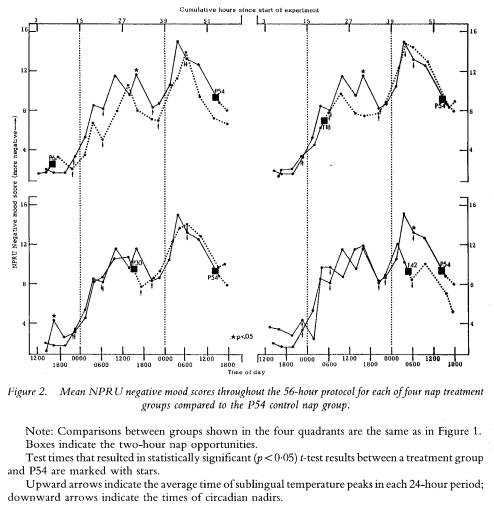
The lack of nap effects on mood parameters cannot be accounted for by the severity of the sleep loss effect on the parameters. As figure 2 shows, NPRU negative mood scores increase over time in all groups with few differences emerging as a function of having napped. Thus, like performance parameters, mood ratings co-varied with sleep loss and circadian phase, but not as a function of obtaining a nap.
The question of whether the early 'prophylactic' naps were more beneficial for performance than later 'recovery' naps was addressed by comparing the data for the combined P6 and T18 groups to the data for the combined P30 and T42 groups. The P ratio for the top four performance variables in table 3 for the combined P6 and T18 groups was 36/56 (68%), while the comparable P ratio for combined P30 and T42 was 18/30 (60%). Although these hybrid percentages do not represent truly independent cases, a proportions test between them was not statistically significant (z = 0.73). It is of interest to note that the comparable proportion for the combined circadian peak naps (P6 and P30) was 60% (29/48), while the value for the circadian trough naps (T18 and T42) was 71% (27/38). Again, a test for the difference between the proportions was not significant (z = 1.02), providing no support for the hypothesis that nap placement differentially affected the performance benefits.
Discussion
The results provide a picture of nap benefits for performance but not for mood, a pattern consistent with the results of some other studies of napping during prolonged wakefulness (Englund et al. 1985, Gillberg 1984, Naitoh et al. 1982). That the effects are general across performance parameters is evidenced in table 3, where the top four variables (those most affected by a nap) are different aspects of each of the four performance tasks. The overall estimate used to show the effects of naps on performance relative to mood is not intended, however, to suggest that the performance measures are independent of each other. Although one task parameter may be somewhat more sensitive to sleep loss and therefore to the benefits of a two-hour sleep period during such a protocol, there is sufficient diversity among the tasks to suggest that the nap effect impacts broadly on the capacity to perform or to carry out work during prolonged wakefulness. Furthermore, the tasks most affected involve a range of performance requirements that are common features of many real-world work scenarios, and thus there is no reason to believe that the data do not generalize to field situations.
148 D. F. Dinges et al.
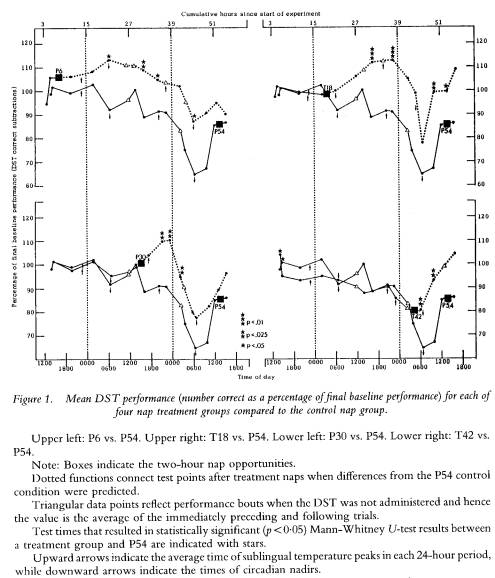
The effects of naps arc limited, however. Overall, only half (48`%,) of predicted differences in performance parameters were statistically significant (see table 2); a pattern that goes well beyond what chance would allow but that falls far short of an ideal effect of 100% on performance parameters (i.e. all performance variables enhanced by a nap for the entire duration of time between each nap and the 54 nap). This suggests that there is only so much benefit from a two-hour sleep opportunity nested within a 56-hour period otherwise devoid of sleep. As the data in figure 1 reveal, the performance benefit may not be immediately evident, especially if the nap is taken before sleep loss (upper half), and the benefit may not last for more than 12 hours if the nap is taken after sleep loss (lower half). Thus, the benefit of a nap might not be evident in a study that measured performance only once or twice, especially if the performance parameter used was one that was variable due to motivational or measurement factors.
149 The benefits of napping
Both the lapsing seen on task-paced psychomotor measures (e.g. VRT slowest 10%) as a function of intense sleepiness, as well as the slowing seen on subject-paced cognitive measures (e.g. MAST speed on two-item target), benefited from a nap. This is important because these tasks have been used in field studies of sleep loss and shift work (cf. Glenville and Wilkinson 1979, Folkard et al. 1976). The benefits were not equally great for all measures, however. A nap improved VRT lapsing more often than any other measure, but not enough to maintain the performance at baseline levels. On the other hand, a nap enhanced DST cognitive performance less often, but the gains seemed to hold the performance near baseline levels for a period of time. These differences in the magnitude of the benefit at any time point versus the length of the benefit across time points appear to reflect differential learning curves of tasks and differential sensitivity to sleep loss. The longer (temporally) the learning curve (e.g. for cognitive tasks) the more likely the performance during prolonged work will hover around 100% of baseline. Similarly, the more sensitive a task is to sleep loss (e.g. VRT slowest 10%), the less likely that a short period of sleep will return performance on it to baseline levels.
150 D. F. Dinges et al.
In terms of the temporal placement of the nap within the protocol, the results for cognitive task parameters presented here are fully consistent with those reported earlier for psychomotor performance (Dinges et al. 1987). Performance is benefited no matter when the nap takes place. Particularly noteworthy in this regard, however, is the fact that early naps (i.e. P6 and T18), those Orne has described as 'prophylactic' (Evans and Orne 1976) in prolonged work scenarios, produced improved performance for many hours, despite the fact that they involved quantitatively less sleep and qualitatively less deep sleep than later naps (Dinges 1986). In terms of relative performance benefits, therefore, the depth of the sleep after prolonged prior wakefulness was considerably less important than simply being able to obtain sleep prior to prolonged wakefulness. The implication is that warding off sleepiness or sleep debt accumulation by napping in advance of its build-up during a prolonged wakefulness scenario is as beneficial as attempting to pay it off later by napping deeply.
The failure to find a beneficial effect of a nap on any of the 24 mood parameters investigated is striking; all the more because the mood scales used involve many parameters that are among the most sensitive to sleep loss and circadian variation. In one sense this provocative finding strengthens the validity of the performance results. If the performance effects had been associated with differential incentive on the part of nap groups to appear more alert in accordance with the hypothesis, mood self-report measures should also have evidenced benefits from the naps. Rather than responding to implicit experimental demand characteristics (Orne 1962) that naps would help them, subjects seemed to be phenomenally unaware of their improved performance following a nap. This suggests that mood reports in general, and sleepiness/fatigue reports in particular, are a less sensitive index of nap effects in sustained wakefulness scenarios. Why this should be so remains unclear. It could be that subjective fatigue is fundamentally different from other behavioural expressions of sleepiness (Broughton 1982), or that it is more profoundly influenced by contextual variables (Dement and Carskadon 1982, Dinges in press), or that it is more sensitive to sleep loss than performance and hence is less readily affected by a small amount of sleep nested in a prolonged wakefulness scenario. While changes in some mood ratings might require more sleep than the two-hour nap opportunity provided, the lack of nap effects on such ratings cannot be attributed to the self-reports reaching maximum levels or asymptoting early in the protocol. There is no evidence the mood ratings were changing more dramatically or rapidly than were some performance measures; this is only an impression, however, since a different metric was used for the former than for the latter.
Although most studies have not observed nap effects on mood variables (Englund et al. 1985, Gillberg 1984, Naitoh et al. 1982), two investigations of field exercises in military personnel have noted nap benefits for both performance and mood (Haslam 1985, Opstad et al. 1978). It is possible that other variables in these field environments, such as withdrawing from the physical demands of the workload when taking a nap or knowledge that a nap is imminent (cf. Haslam 1983), contributed to these mood findings.
Although a single two-hour nap, taken any time during 56 hours of sustained wakefulness with intermittent work demands, clearly appeared to aid performance relative to no sleep, the benefit was not sufficient either to maintain performance at baseline levels or to enhance mood. There is little doubt that more sleep, in the form of longer or more frequent sleep opportunities, would be required for such optimal effects. Nevertheless, it is striking how much performance benefit is derived from two hours sleep out of 56 hours, even if subjects are not phenomenally aware of the benefit. In terms of the question posed in the introduction, the results suggest that a nap at any time can be beneficial for performance after the transient few minutes of post-sleep disorientation ('sleep inertia') wears off. The sooner one naps in such prolonged work protocols, the more likely that sleepiness effects on performance will be delayed.
151 The benefits of napping
This study focused on two-hour naps, rather than longer periods of sleep and/or shift work (i.e. long periods of sleep that are displaced chronobiologically), for three reasons:
(1) much less work has been conducted on napping than on four or more hours of sleep;
(2) napping holds considerable promise for real-world quasi-continuous work scenarios, where sleep opportunities are limited;
(3) nap studies permit evaluations of the temporally confounded effects of prior wakefulness (i.e. sleep loss) and circadian phase.
The results support the notion that napping can be a partially effective countermeasure to the debilitating effects of sleepiness on performance.
Unfortunately, the complexity of behavioural research on sleep, sleep loss, and chronobiology all of which are involved in the kind of study conducted here is often seen as an impediment to understanding and using the results in real-world work scenarios. Nevertheless, we can hardly afford to do otherwise, if we consider:
(1) that all humans get sleepy and eventually sleep (voluntarily or involuntarily);
(2) that this is an internally timed process that happens every day whether we consent to it or not;
(3) that if we resist the pressure it will eventually interfere with our performance at all levels;
(4) that no one can resist it indefinitely.
Seen in this light, sleep is perhaps the most pervasive behavioural control in nature, and there is every reason to believe that the basic process of sleepiness and its effect on functioning are among the most potent variables in situations that require sustained periods of continuous or intermittent performance demands even when highly skilled and dedicated personnel arc involved (Mitler et al. 1988, Dinges in press). Finding ways to cope with the need to sleep and the effects of sleepiness, while work is conducted, is therefore a nontrivial goal.
As more and more work is done around-the-clock, by more workers, in more government and private industries -- especially those involving public health and safety -- sleep loss and chronobiological variance in behaviour take on an even greater importance as two of the most pervasive limitors of human ability. To ignore them is to court disaster: to search for effective strategies, such as napping, that permit the work activity while minimizing the effects of sleepiness is consistent with good planning and management. To be sure, other stressors (e.g. threat to life or catastrophic consequences associated with a human error) also often exist in prolonged work scenarios. Generally, they compound sleep loss effects and make the search for viable countermeasures, such as napping, all the more crucial. There is, therefore, good reason to continue to assert that factors affecting the alertness level of the human brain warrant renewed consideration in a world that increasingly relies upon human work around-the-clock for indefinite periods.
Acknowledgements
This research was supported in part by the US Office of Naval Research contract N0001480-C-0380, in part by grant MH-19156 from the National Institute for Mental Health, and in part by a grant from the Institute for Experimental Psychiatry Research Foundation. The authors gratefully acknowledge the help of Stephen R. Fairbrother, Chris Auxier, Mary F. Auxier, Ann Maliniak Whitehouse, Christine M. Dinges, Richard Barras, Elizabeth Whitehouse, and Jeffrey Moore in conducting this study, and the technical and computer expertise provided by John W. Powell in data reduction and analyses.
152 D. F. Dinges et al.
References
ANGIBOUST, R., and GOUARS, M. (1972). Tentative d'evaluation de l'efficacite operationelle du personnel de l'aeronautique militaire au cours de veilles nocturnes. In W. P. Colquhoun (ed.), Aspects of Human Efficiency: Diurnal Rhythm and Loss of Sleep (English Universities Press: London), pp. 151-170.
ANGUS, R. G., and HESLEGRAVE, R. J. (1985). Effects of sleep loss on sustained cognitive performance during a command and control simulation. Behavior Research Methods, Instruments, & Computers, 17, 55-67.
BROUGHTON, R. (1982). Performance and evoked potential measures of various states of daytime sleepiness. Sleep, 5 Suppl. 2, S135-S146.
DEMENT, W. C., and CARSKADON, M. A. (1982). Current perspectives on daytime sleepiness: The issues. Sleep, 5 Suppl. 2, S56-S66.
DINGES, D. F. (in press). The nature of sleepiness: Causes, contexts, and consequences. In A. Baum and A. S. Stunkard (eds.), Eating, Sleeping and Sex: Perspectives on Behavioral Medicine (L. Erlbaum: Hillsdale, NJ).
DINGES, D. F. (1986). Differential effects of prior wakefulness and circadian phase on nap sleep. Electroencephalography and Clinical Neurophysiology, 64, 224-227.
DINGES, D. F., ORNE, M. T., and ORNE, E. C. (1984). Sleepiness during sleep deprivation: The effects of performance demands and circadian phase. In M. H. Chase, W. B. Webb, and R. WilderJones (eds.), Sleep Research, Vol. 13 (University of California: Los Angeles), p. 189.
DINGES, D. F., ORNE, M. T., and ORNE, E. C. (1985). Assessing performance upon abrupt awakening from naps during quasi-continuous operations. Behavior Research Methods, Instruments, and Computers, 17, 37-45.
DINGES, D. F., ORNE, M. T., WHITEHOUSE, W. G., and CAROTA ORNE, E. (1987). Temporal placement of a nap for alertness: contributions of circadian phase and prior wakefulness. Sleep, 10, 313329.
DINGES, D. F., and POWELL, J. W. (1985). Microcomputer analyses of performance on a portable, simple visual RT task during sustained operations. Behavior Research, Methods, Instruments and Computers, 17, 652-655.
DINGES, D. F., and WHITEHOUSE, W. G. (1985). A dual-probe recognition memory tasks for use during sustained operations. Behavior Research Methods, Instruments, and Computers, 17, 656-658.
ENGLUND, C. E., RYMAN, D. H., NAITOH, P., and HOGDON, J. A. (1985). Cognitive performance during successive sustained physical work episodes. Behavior Research Methods, Instruments, and Computers, 17, 75-85.
EVANS, F. J., and ORNE, M. T. (1976 a). Recovery from fatigue. US Army Medical Research and Development Command Report No. 60. (US Army Medical Research and Development Command: Washington, DC). (DTIC No. A100347.)
EVANS, F. J., and ORNE, M. T. (1976b). Recovery from fatigue. US Army Medical Research and Development Command Report No. 65. (US Army Medical Research and Development Command: Washington, DC). (NTIS No. A100310.)
FOLKARD, S., KNAUTH, P., MONK, T. H., and RUTENFRANZ, J. (1976). The effect of memory load on the circadian variation in performance efficiency under a rapidly rotating shift system. Ergonomics, 19, 479-488.
GILLBERG, M. (1984). The effects of two alternative timings of a one-hour nap on early morning performance. Biological Psychology, 19, 45-54.
GLENVILLE, M., BROUGHTON, R. J., WING, A. M., and WILKINSON, R. T. (1978). Effects of sleep deprivation on short duration performance measures compared to the Wilkinson auditory vigilance task. Sleep, 1, 169-176.
GLENVILLE, M., and WILKINSON, R. T. (1979). Portable devices for measuring performance in the field: The effects of sleep deprivation and night shift on the performance of computer operators. Ergonomics, 22, 927-933.
HASLAM, D. R. (1981). The military performance of soldiers in continuous operations. In L. C. Johnson, D. I. Tepas, W. P. Colquhoun and M. J. Colligan (eds.), Biological Rhythms, Sleep and Shift Work (SP Medical & Scientific Books: New York), pp. 217-230.
HASLAM, D. R. (1983). The incentive effect and sleep deprivation. Sleep, 6, 4, 362-368.
HASLAM, D. R. (1985). Sleep deprivation and naps. Behavior Research Methods, Instruments, and Computers, 17, 46-54.
153 The benefits of napping
HERSCOVITCH, J., and BROUGHTON, R. (1981). Performance deficits following short-term partial sleep deprivation and subsequent recovery oversleeping. Canadian Journal of Psychology, 35, 4, 309322.
HODDES, E., ZARCONE, V., SMYTHE, H., PHILLIPS, R., and DEMENT, W. C. (1973). Quantification of sleepiness: A new approach. Psychophysiology, 10, 4, 431-436.
JOHNSON, L. C., and NAITOH, P. (1974). The Operational Consequences of Sleep Deprivation and Sleep Deficit. NATO/AGARDograph No. 193. (Technical Editing and Reproduction: London.)
LISPER, H.-O., and KJELLBERG, A. (1972). Effects of 24-hour sleep deprivation on rate of decrement in a 10-minute auditory reaction time task. Journal of Experimental Psychology, 96, 2, 287-290.
LUMLEY, M., ROEHRS, T., ZORICK, F., LAMPHERE, J., and ROTH, T. (1986). The alerting effects of naps in sleep-deprived subjects. Psychophysiology, 23, 403-408.
MCNAIR, D. M., LORR, M., and DRUPPLEMAN, L. F. (1971). EITS Manual for the Profile of Mood States (Educational and Industrial Test Services: San Diego).
MITLER, M., CARSKADON, M. A., CZEISLER, C., DEMENT, W. C., DINGES, D. F., and GRAEBER, R. C. (1988). Report of the committee on catastrophes, sleep and public policy of the Association of Professional Sleep Societies. Sleep, 11, 100-109.
MORGAN, B. B. (1974). Effects of continuous work and sleep loss in the reduction and recovery of work efficiency. American Industrial Hygiene Association journal, 35, 13-20.
NAITOH, P. (1981). Circadian cycles and restorative power of naps. In L. C. Johnson, D. I. Tepas, W. P. Colquhoun, M. J. Colligan (eds.), Biological Rhythms, Sleep and Shift Work (SP Medical & Scientific Books: New York), pp. 553-580.
NAITOH, P., ENGLUND, C. E., and RYMAN, D. (1982). Restorative power of naps in designing continuous work schedules. Journal of Human Ergology (Tokyo), 11 (Suppl), 259-278.
NICHOLSON, A. N., PASCOE, P. A., ROEHRS, T., et al. (1985). Sustained performance with short evening and morning sleeps. Aviation Space and Environmental Medicine, 56, 105-114.
OPSTAD, P. K., EKANGER, R., NUMMESTAD, M., and RAABE, N. (1978). Performance, mood, and clinical symptoms in men exposed to prolonged, severe physical work and sleep deprivation. Aviation Space and Environmental Medicine, 49, 1065-1073.
ORNE, M. T. (1962). On the social psychology of the psychological experiment: With particular reference to demand characteristics and their implications. American Psychologist, 17, 11, 776-783.
TEPAS, D. I. (1980). The assessment of fatigue and tension. Unpublished manuscript.
THAYER, R. E. (1967). Measurement of activation through self-report. Psychological Reports, 20, 663-678.
THAYER, R. E. (1986). Activation-Deactivation Adjective Check List: Current overview and structural analysis. Psychological Reports, 58, 607-614.
THORNE, D., GENSER, S., SING, H., and HEGGE, F. (1983). Plumbing human performance limits during 72 hours of high task load. In S. E. Forshaw (ed.), Proceedings of the 24th defense research group seminar on the human as a limiting element in military systems: Vol. 1. NATO-DRG Report No. DS-A-DR(83) 170, (NATO Defence Research Group: Toronto), pp. 17-40.
TILLEY, A. J., and WILKINSON, R. T. (1984). The effects of a restricted sleep regime on the composition of sleep and on performance. Psychophysiology, 21, 406-412.
TILLEY, A. J., WILKINSON, R. T., WARREN, P. S. G., WATSON, B., and DRUD, M. (1982). The sleep and performance of shiftworkers. Human Factors, 24, 629-641.
WILKINSON, R. T., and HOUGHTON, D. (1982). Field test of arousal: A portable reaction timer with data storage. Human Factors, 24, 487-493.
The preceding paper is a reproduction of the following
article (Dinges, D. F., Whitehouse, W. G., Orne, E. C., & Orne, M. T. The
benefits of a nap during prolonged work and wakefulness. Work & Stress,
1988, 2, 139-153.). It is reproduced here with the kind permission of Taylor
and Francis. http://www.tandf.co.uk/journals
.