Atomic
Quantum Mechanics:
Visualizing
the Wave Function
 
Introduction:
A fundamental principle of quantum mechanics (the Heisenberg uncertainty
principle) tells us that it is not possible to simultaneously know the
exact position and velocity of a particle. As a result, we
can never describe the state of an electron as being at a particular location
at a certain time. The best we can do is to specify the "wave function"
for the electron.
The meaning
of the wave function, 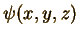 ,
is that the absolute value squared of the wave function, ,
is that the absolute value squared of the wave function, 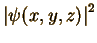 ,
gives the probability of finding the electron at position (x,y,z).
The absolute value squared is always positive or zero, as probabilities
must be. ,
gives the probability of finding the electron at position (x,y,z).
The absolute value squared is always positive or zero, as probabilities
must be.
The
wave function itself can be positive or negative (in fact, it can also
equal a complex number), so that two wave functions can add constructively
or destructively. If wave functions on neighboring atoms have the
same sign, they add constructively (the sum is bigger than either of the
individual wave functions) and this makes a bonding orbital. If the
wave functions have opposite signs, they combine destructively (the sum
is smaller than the individual wave functions) and this makes an anti-bonding
orbital. We will learn more about how the combination of wave functions
gives rise to chemical bonding interactions in the next chapter.
-
You can
run an interactive JAVA applet, which allows you to choose an atomic configuration
and calculate the wave functions for each electron in the atom.
-
You can
visit topic pages to help you master the concepts of atomic quantum mechanics.
-
Each page
poses various questions to enhance your understanding. Many of these
questions prompt you to run the JAVA applet to help you answer questions
about atomic quantum mechanics.
Objectives:
The main goal of this module is to enrich your
understanding
of this important scientific principle through explanation,
illustration,
and exploration. As a result of using this module, you will be
able
to achieve these objectives:
-
to explain
what a wave function is by defining it in your own words
-
to explain
what a radial node is by defining it in your own words
-
to explain
what an angular node is by defining it in your own words
-
to explain
Heisenberg uncertainty by defining it in your own words
-
to illustrate
how wave functions change when atoms are ionized by computing wave functions
for various electronic configurations and describing each result
-
to illustrate
how wave functions with different principal and angular momentum quantum
numbers differ by graphing various wave functions and describing the results
-
to analyze
how wave functions for different atoms differ by solving for wave functions
of different atoms and comparing the result
-
to analyze
how excited and ionized atomic configurations affect the wave functions
by solving for wave functions in various configurations and comparing the
results
-
to synthesize
the results of your explorations by writing an essay of your own description
of atomic quantum mechanics, including your analysis of the effects of
ionization, excitation, and change of element on the electronic wave functions.
Getting
Started: You can
select your own way to use this
module.
Visit the topic pages by using the outline below or the arrow
buttons
above. (Topic pages will be online soon!) Explore freely by
running the applet on your own, or use the applet to help you answer some
of the questions on the topic pages. It's up to you!
© Andrew M. Rappe
|
