Judgment and Decision Making, vol. 5,
no. 6, October 2010, pp. 428-436
To give or not to give: Parental experience and adherence to the Food and Drug Administration warning about over-the-counter cough and cold medicine usageTalya Miron-Shatz* 1,2,
Greg Barron3,
Yaniv Hanoch4,
Michaela Gummerum4,
Glen M. Doniger1
1 Ono Academic College, Israel
2 University of Pennsylvania
3 Negotiation.com
4 Plymouth University, England |
The Food and Drug Administration (FDA) warned against administering
over-the-counter cough and cold medicines to children under 2. This
study evaluated whether experienced parents show poorer adherence to
the FDA warning, as safe experiences are predicted to reduce the
impact of warnings, and how adherence can be improved. Participants
included 218 American parents (mean age: 29.98 (SD = 6.16), 82.9%
female) with children age ≤ 2 who were aware of the FDA
warning. We compared adherence
among experienced (N=142; with other children
> age 2) and inexperienced parents (N=76; only
children ≤2). We also evaluated potential moderating variables (amount
of warning-related information received, prevalence of side effects,
trust in the FDA, frequency of coughs and colds, trust in drug
packaging) and quantified the impact of amount of
information. Logistic regression assessed the ability of experience
alone, and experience combined with amount of information, to predict
adherence. 53.3% of inexperienced but 28.4% of experienced parents
were adherent (p = 0.0003). The groups did not differ on
potential moderating variables. Adherence was 39.5% among
experienced parents receiving “a lot of information”, but 15.4%
for those receiving less (p = 0.002); amount of information
did not affect adherence in inexperienced parents (p =
0.22) but uniquely predicted adherence compared to a model with
experience alone (p = 0.0005). Experienced parents were
also less likely to mistrust drug packaging (p =
0.03). Targeting FDA information to experienced parents,
particularly via drug packaging, may improve their adherence.
Keywords: OTC-CCM, adherence, experience, parent, decision-making, risk
assessment, FDA warning, compliance, young children.
1 Introduction
In October 2007, following an expert review regarding the safety of
over-the-counter cough and cold medication (OTC-CCM), the Food and Drug
Administration (FDA) recommended that “these drugs
not be used to treat infants and children under 2 years of age because
serious and potentially life-threatening side effects can
occur.” (United States Food and Drug Administration
[FDA], 2008). Since earlier studies have shown that people ascribe
little risk or harm to OTC medications (Glasziou, 2002; Roumie &
Griffin, 2004), it is important to evaluate adherence to the FDA
warning. Indeed, despite wide publicity, a recent nationally
representative survey found that only 16% of parents with children
under 2 (or 19% of parents who were aware of the FDA warning) intended
to comply with the FDA warning (National Public Radio/Kaiser Family
Foundation/Harvard School of Public Health [NPR/KFF/HSPH], 2007).
The decision-making literature suggests people are ill-equipped to
incorporate information about rare side effects into their decisions
(Barron & Erev, 2003; Erev & Barron, 2005; Fox & Hadar, 2006;
Hertwig, Barron, Weber, & Erev, 2004; Kahneman & Tversky, 1979; Li,
Rakow, & Newell, 2009). When information about a rare event comes
externally from a description, as from a warning, people tend to
overweigh the rare event in their decisions (Barron & Erev, 2003; Li,
Rakow, & Newell, 2009). In other words, people behave as if the rare
event is more likely to occur than its objective
probability. Conversely, when people learn from their own experiences,
they tend to underweigh rare events, behaving as if the event is less
likely to occur than its objective probability (Barron & Erev, 2003;
Erev & Barron, 2005; Fox & Hadar, 2006; Hertwig et al., 2004; Li et
al., 2009).1
The two tendencies imply that different decisions can result from the
same information, depending upon how one learns about the potential
consequences of the choices and their probabilities. A recent
literature has focused on the mechanisms underlying this apparent
“gap” between description- and experience-based decisions and their
relative importance (Barron & Yechiam, 2009; Camilleri & Newell,
2009; Fox & Hadar, 2006; Fox & Hadar, 2009; Hertwig et al.,
2004). The mechanisms include 1) recency — when rare events happen
in the distant past and memory is constrained, decisions will rely on
a small set of past outcomes, 2) statistical sample bias — as rare
events are underrepresented more often than overrepresented (due to
the skewness of the binomial distribution), and 3) judgment bias —
as people incorrectly estimate an event’s likelihood even in an
unbiased sample.
In practice, people gain information from both external
descriptions and personal experience. In deciding whether to adhere to
a given warning, people who have previously used the risk-causing
agent are subject to potentially conflicting influences from the
warning and past experience. Others, possessing little or no
experience with the risk-causing agent, are presumably more reliant
upon the warning. Research has shown that inertia tends to guide the
risk-taking behavior of people who have had safe experiences with the
risk-causing agent, such that they continue their exposure to the
agent despite new information about associated dangers (Barron,
Leider, & Stack, 2008). The FDA warning created an
opportunity to examine this behavior as applied to a risk-causing
agent that affects millions of parents and children, with clear
implications for health policy. By measuring adherence in parents with
older children, who presumably have more experience with colds and use
of OTC-CCM in children under 2, and those without older children, we
were able to test the following hypothesis: behavioral inertia due to
safe experience with OTC-CCM would reduce adherence to the FDA
warning.
Even if a warning with the appropriate content reaches and is understood
by its target audience, recipients may still not know what to believe
if communicators are perceived to have a vested interest (Morgan,
Fischhoff, Bostrom, & Atman, 2002). In the United Kingdom, for
example, parents’ decisions not to vaccinate against
measles, mumps, and rubella (MMR) — despite assurances and campaigns by
the UK government — stemmed largely from lack of trust in messages
about the safety of these vaccines (Cassidy, 2006; Cassidy, Cresswell,
Wilson, & Panter-Brick, 2006; Hobson-West, 2007). Thus, in order to
isolate the effect of experience on adherence, we also measured
parents’ stated trust in the FDA as a source of
information.
We conducted a survey to explore the hypothesis that experienced parents
would exhibit poorer adherence, as well as to examine other factors
potentially affecting adherence: amount of relevant information
received, prevalence of side effects, trust in the FDA, and frequency
of child’s coughs and colds. To our knowledge, this is
the first study to evaluate the effect of parental experience on
adherence to the FDA warning. On the basis of our findings, we propose
a tentative strategy for increasing adherence.
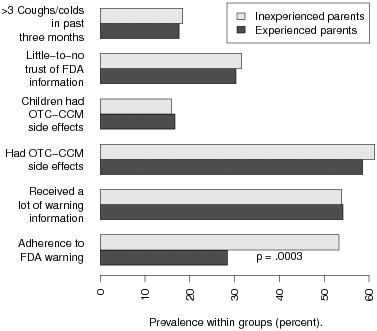
| Figure 1: Prevalence of adherence to the FDA warning against use of OTC-CCM in
young children, separately for experienced (older children in addition to a child
age 2 or younger) and inexperienced (only a child age 2 or younger) parents.
Inexperienced parents were significantly more likely to adhere. However, the groups
were similar in reported amount of warning information received, prevalence of
side-effects in the parents and their children, trust of FDA information, and number
of coughs or colds in the preceding three months. Differences are
non-significant unless indicated. |
2 Methods
Participants (N=218) were parents (mean age [SD]: 29.98 [6.16]
years) of children age 2 and younger who indicated in an online survey
that they had heard of the FDA warning against using OTC-CCM in young
children; parents who reported not having heard of the FDA warning
(N=32) were excluded from analysis. Most participants were
female (82.9%), and most had completed college (63.1%).
Participants were recruited online between January and April 2008 via
paid advertisements on Google and Facebook and completed an online
survey regarding their use and perception of OTC-CCM. All participants
were from the United States, and representation by region (Northeast:
24.4%; Midwest: 27.2%; South: 30.4%; West: 18.0%) was similar to
that as measured by the 2000 US census (United States Census Bureau,
2001). Upon completion of the survey, participants were entered into a
raffle to win one $50 gift certificate for a major Internet retailer.
The primary study outcome was adherence to the FDA warning, as
measured by participants’ reported intention to stop
administering OTC-CCM (Table 1). To quantify the effect of parenting
experience upon adherence, participants were divided into two
groups: experienced parents (N=142; mean age [SD]:
31.09 [5.64] years; 88.6% female; 60.7% completed college), who
reported having older children (in addition to a child or children aged
2 or younger); and inexperienced parents (N=76; mean
age [SD]: 27.93 [6.59] years; 72.4% female; 67.6% completed college),
who reported having only a child aged 2 or younger (Table 1).
Experienced parents were older (p<0.001) but similar
to inexperienced parents in educational level (p=0.12). Other
variables of interest included subjective amount of information
received about the FDA warning, prevalence of OTC-CCM-related side
effects experienced by participants and their children, level of trust
in the FDA and drug packages to provide accurate information on the
safety and effectiveness of OTC-CCM, and occurrence of coughs or colds
in the previous three months (Table 1). These variables were recoded as
dichotomous (Table 1) for consistency with study hypotheses and in
consideration of the small sample size for several response options
(Table 2), as well as to simplify interpretation of their
correspondence with the binary adherence and experience variables.
Frequency of response for each original response option is shown in
Table 2, separately for experienced and inexperienced parents.
The χ2 statistic was used to compare adherence between
experienced and inexperienced parents and to contrast the groups on
other potential moderating variables. The χ2 statistic was also
computed to evaluate the effect of amount of information received upon
each experience group. Logistic regression analysis was used to
evaluate the ability of parental experience alone and combined with
each potential moderating variable to predict adherence; an
interaction term was included to control for moderating effects on
experience. The χ2 statistic was used to assess the unique
contribution of information received.
3 Results
More than half (53.3%) of inexperienced parents adhered to the FDA
warning, compared with just over a quarter (28.4%) of experienced
parents (χ2(1) = 13.08, p = 0.0003; Figure 1).
Parenting experience was a significant predictor of adherence
(B = 1.06, Wald(1) = 12.70, p = 0.0004),
such that inexperienced parents were 2.89 times more likely to be
adherent. The effect of experience could not be attributed to the
experienced group receiving less information, as similar proportions
of both groups reported receiving “a lot of information”
(experienced: 54.2%; inexperienced: 53.9%; χ2(1) = 0.002,
p = 0.97; Figure 1), and experience remained a significant
predictor even when amount of information received and its interaction
with experience were included in the model (B = 1.53,
Wald(1) = 10.07, p = 0.002). Similarly, the effect
could not be attributed to a higher prevalence of OTC-CCM-related side
effects encountered by inexperienced parents (experienced: 58.6%;
inexperienced: 61.3%; χ2(1) = 0.155, p = 0.69) or
their children (experienced: 16.7%; inexperienced: 15.9%;
χ2(1) = 0.018, p = 0.89; Figure 1) as experience
continued to predict adherence even after inclusion of each potential
moderator and its interaction with experience in the model (parents:
B = 1.00, Wald(1) = 4.01, p = 0.045;
children: B = 1.10, Wald(1) = 10.56, p =
0.001). The experience effect could not be accounted for by greater
mistrust in the FDA in the experienced group, as the proportion of
parents expressing little to no trust in FDA information was
comparable among the groups (experienced: 30.3%; inexperienced:
31.6%; χ2(1) = 0.039, p = 0.84; Figure 1), and
experience again remained a significant predictor even after trust of
FDA and its interaction with experience were included in the model
(B = 1.61, Wald(1) = 8.08, p =
0.004). Finally, poorer adherence in the experienced group did not
appear to be explained by more coughs or colds, as the frequency of
parents reporting “more than several” coughs or colds in the months
prior to the survey was similar between the groups (experienced:
17.6%; inexperienced: 18.4%; χ2(1) = 0.022, p = 0.88;
Figure 1). Experience was no longer a significant predictor of
adherence after inclusion of this potential moderator and its
interaction with experience in the logistic regression model
(B = 0.52, Wald(1) = 0.28, p = 0.59), but
it remained significant after inclusion as a covariate in a parametric
analysis without an interaction term (F(1,177)=13.65,
p=0.0003; see below).
Parametric analyses run on the original variables (i.e., using all the
response options shown in Table 1, prior to recoding) were highly consistent with
the above, yielding an experience effect on adherence
(F(1,178)=13.15, p=0.0004) and no differences among
the experience groups on potential moderators (information:
F(1,216)=0.30, p=0.58; side effects in parents:
F(1,213)=0.47, p=0.50; side effects in children:
F(1,205)=0.03, p=0.86; trust of FDA:
F(1,216)=1.01, p=0.32; coughs and colds:
F(1,216)=0.59, p=0.44). Moreover, the significant
experience effect was preserved (p<0.003) even
after covarying for each of the potential moderators. None of the
potential moderating variables was significantly correlated with both
adherence and experience. Results were comparable irrespective of
whether the “I am not sure what I will do” adherence response was
included.
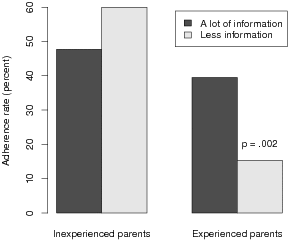
| Figure 2: Adherence rate among parents who reported receiving a lot of information
about the FDA warning as compared with those who reported receiving less
information, separately for experienced and inexperienced parents. Receipt of more
information significantly improved adherence rate in experienced parents, but not in
inexperienced parents. Differences are non-significant unless indicated. |
| Table 1: Study variables, associated survey questions and responses, and
coding scheme for statistical analysis. |
Variable(s) | Survey question | Survey responses | Coding |
Adherence | In view of the FDA recommendation, what do you intend to do when your
child has a cough or cold in the future? | a) I intend to keep giving my children the cough and cold medicine we
have at home and buy more when we run out. b) I intend to keep giving my children the cough and cold medicine we
have at home, but to reconsider using them when we run out. c) I intend to keep giving my children the cough and cold medicine we
have at home, but I will not buy more when we run out. d) I intend to stop giving my children cough and cold medicine e) I am not sure what I will do | adherent: response d)
non-adherent: all other responses |
Experience | Please list the ages of ALL of your children. (Leave the extra boxes
blank if you have fewer than 8 children; if you have more than 8
children, please list the ages of the 8 YOUNGEST.) | age of: YOUNGEST child: __ 2nd youngest child: __ 3rd youngest child: __ … 8th youngest child: __ | experienced: older children in addition to the one child ≤2
inexperienced: only one child ≤2 |
Amount of information received about FDA warning | Have you heard that a panel of expert advisers for the FDA reported that
most over-the-counter cough and cold medicines do not work for children
under 6, and may also be dangerous if taken in quantities that are too
large, as a result of which their use cannot be recommended? | a) I have not heard about this report at all* b) I have not heard much about this report c) I have heard something about this report d) I have heard a lot about this report | a lot: response d)
not a lot: responses b) and c) |
Prevalence of Side Effects in Participants | How often have YOU experienced side effects after taking an
over-the-counter cough and cold medicine? | a) never b) rarely c) sometimes d) often e) always | yes: responses b) through e)
no: response a) |
Prevalence of side effects in children | Think of the over-the-counter cough and cold medicines that you gave
your child when he/she had a cough or a cold during the past three
months. How often has your child experienced side effects after taking
any of these medications? | a) never b) rarely c) sometimes d) often e) always | yes: responses b) through e)
no: response a) |
Trust of FDA; Trust of medicine packages | How much do you trust each of the following sources to provide you with
accurate information about the safety and effectiveness of
over-the-counter medicines for your children: the Food and Drug
Administration (FDA), the information included in the over-the-counter
medicine packages? | a) not at all b) a little c) somewhat d) a lot | little-to-none: responses a) and b)
somewhat-to-a-lot: responses c) and d) |
Number of coughs or colds in previous three months | Think of the last three months. Can you tell us how many times your
child had a cough or a cold during this period? | a) 0 times b) 1–3 times c) 4–6 times d) 6–10 times e) more than 10 times | 0–3 times: responses a) and b)
>3 times: responses c) through e) |
| * Respondents who had not heard about the FDA report at
all were excluded from this study. |
| Table 2: Frequency of responses to study variables for experienced
(N=142) and inexperienced (N=76) parents. |
| | | % Participants (n) |
Variable | Survey responses | Experienced | Inexperienced |
Adherence | I intend to keep giving my children the cough and cold medicine we
have at home and buy more when we run out | 39.4 (56) | 19.7 (15) |
| | I intend to keep giving my children the cough and cold medicine we
have at home, but to reconsider using them when we run
out | 11.3 (16) | 11.8 (9) |
| | I intend to keep giving my children the cough and cold medicine we
have at home, but I will not buy more when we run out | 2.8 (4) | 1.3 (1) |
| | I intend to stop giving my children cough and cold
medicine | 28.2 (40) | 51.3 (39) |
| | I am not sure what I will do | 17.6 (25) | 14.5 (11) |
| | (no response) | 0.7 (1) | 1.3 (1) |
21inAmount of information received about FDA warning | I have not heard about this report at
all1 | - | - |
| | I have not heard much about this report* | 9.2 (13) | 3.9 (3) |
| | I have heard something about this report | 36.6 (52) | 42.1 (32) |
| | I have heard a lot about this report | 54.2 (77) | 53.9 (41) |
21inPrevalence of side effects in participants | never | 40.8 (58) | 38.2 (29) |
| | rarely | 33.1 (47) | 35.5 (27) |
| | sometimes | 21.1 (30) | 15.8 (12) |
| | often | 3.5 (5) | 9.2 (7) |
| | always | 0 (0) | 0 (0) |
| | (no response) | 1.4 (2) | 1.3 (1) |
21inPrevalence of side effects in children | never | 81.0 (115) | 76.3 (58) |
| | rarely | 11.3 (16) | 9.2 (7) |
| | sometimes | 4.9 (7) | 3.9 (3) |
| | often | 0 (0) | 1.3 (1) |
| | always | 0 (0) | 0 (0) |
| | (no response) | 2.8 (4) | 9.2 (7) |
Trust of FDA | not at all | 8.5 (12) | 6.6 (5) |
| | a little | 21.8 (31) | 25.0 (19) |
| | somewhat | 50.0 (71) | 36.8 (28) |
| | a lot | 19.7 (28) | 31.6 (24) |
21inTrust of medicine packages | not at all | 7.7 (11) | 18.4 (14) |
| | a little | 32.4 (46) | 36.8 (28) |
| | somewhat | 46.5 (66) | 39.5 (30) |
| | a lot | 13.4 (19) | 5.3 (4) |
21inNumber of coughs or colds in previous three months | 0 times | 4.9 (7) | 7.9 (6) |
| | 1–3 times | 77.5 (110) | 73.7 (56) |
| | 4–6 times | 15.5 (22) | 10.5 (8) |
| | 6–10 times | 0.7 (1) | 2.6 (2) |
| | more than 10 times | 1.4 (2) | 5.3 (4) |
| * Respondents who had not heard about the FDA report at
all were excluded from this study. |
To explore whether adherence by experienced parents might be improved
through increased awareness of the FDA warning, the effect on
adherence of amount of information received was examined in each
group. Indeed the adherence rate (39.5%) in experienced parents who
reported receiving “a lot of information” was more than twice the
rate (15.4%) in those who reported receiving less information
(χ2(1) = 10.01, p = 0.002; Figure 2). By comparison,
amount of information did not affect adherence rate in the
inexperienced group (a lot: 45.7%; less: 60.0%; χ2(1) = 1.53,
p = 0.22; Figure 2).2
Moreover, in a logistic regression model including an interaction
term, both amount of information (B = 1.28, Wald(1)
= 9.41, p = 0.002) and experience (B = 1.53,
Wald(1) = 10.07, p = 0.002) predicted adherence,
such that parents who reported receiving “a lot of information” were
3.59 times more likely to be adherent than their counterparts who
received less information, and inexperienced parents were 4.63 times
more likely to adhere than experienced parents. The interaction term
did not reach significance (B = -0.70, Wald(1) =
1.25, p = 0.26), possibly due to insufficient sample
size.3 Amount of
information (and its interaction with experience) contributed uniquely
relative to experience alone (χ2(1) = 11.94, p =
0.0005), suggesting that increasing the amount of information may
boost adherence irrespective of the experience effect.
Finally, to give an indication of the efficacy of using drug packaging
to increase awareness of the FDA warning in experienced parents, trust
in drug packaging information was compared across groups. In fact,
mistrust of medicine packaging information was significantly more
prevalent among inexperienced (55.3%) than experienced (40.1%)
parents (χ2(1) = 4.57, p = 0.03),
suggesting that experienced parents might indeed be receptive to
additional information provided via packaging.4
4 Discussion
The current findings suggest that experienced parents exhibit reduced
adherence to the FDA warning against giving OTC-CCM to children under
2. Furthermore, reduced adherence in experienced parents does not
appear attributable to receiving less information, fewer side effects,
greater mistrust in FDA, or fewer coughs and colds. Thus the effect
seems consistent with the experienced parents’
behavioral inertia (Barron et al., 2008), motivated by a history of
safe experiences with OTC-CCM. Despite poorer adherence overall,
experienced parents who received “a lot of
information” about the warning were more adherent than
those who did not, and amount of information uniquely predicted
adherence. Thus, experienced parents’ behavioral
inertia may be counteracted by providing them with additional
information. Finally, the data suggest that drug packaging rather than
public announcements may be a particularly effective medium for
increasing adherence in experienced parents.
The FDA advisory committee’s public announcement of the
OTC-CCM warning (FDA, 2008) was effective, since the majority of both
experienced and inexperienced parents reported receiving
“a lot of” warning-related
information (Figure 1). Despite similar intake of information between
the groups, adherence was much poorer in the experienced parents
(Figure 1). Thus it would seem that even more information and/or more
effective dissemination of it must be used to combat the behavioral
inertia in experienced parents. Fortunately, experienced parents do
show better adherence when given additional information (Figure 2) and
generally trust the FDA (as do inexperienced parents; Figure 1).
Moreover, experienced parents tend to trust drug packaging, suggesting
that this medium may be employed to boost adherence. Indeed our data
suggest that levels of trust in drug packaging and in the FDA are
comparable. These findings, coupled with the immediacy and efficiency
associated with drug package warnings may make them a viable
alternative to public announcements.
Inexperienced and experienced parents reported similar prevalence of
OTC-CCM side effects in themselves and their children, though with
higher prevalence in the parents than in the children (Figure 1).
Notably, this observation may not reflect reduced likelihood of OTC-CCM
side effects in children relative to adults for a given OTC-CCM
administration. Rather, this difference may be explained by parents
being older and hence having had more opportunities to experience side
effects.
Several limitations of the present study should be considered. First of
all, collection of data by self-report rather than by objective
observation may have artificially increased some ratings (i.e.,
adherence, amount of information) and lowered others (i.e., mistrust of
FDA and packaging), given considerations of social desirability
(Schwarz, 2007). However, such influences would be expected to affect
all participants equally, irrespective of parenting experience. Second,
other variables that may moderate adherence were not examined (e.g.
physician’s advice, media influence).
Third, improved adherence with greater information in experienced (but
not inexperienced) parents may be an artifact of greater impact of
information at lower levels of adherence. Further studies should
examine the effect of provision of additional information over the
range of adherence values. To describe and track effects of behavioral
inertia over time, longitudinal studies are ultimately required.
Finally, the survey’s online format may raise concerns
about selection issues. Though the web has been identified as an
excellent tool for generating large, heterogeneous samples (Birnbaum,
2004), participants in the present study were slightly younger and more
highly educated than the general US population (see 2000 US Census;
United States Census Bureau, 2001), highlighting the need for larger,
more representative studies.
Our results have clear public health implications, as continued use of
OTC-CCM may increase the risk of serious side effects. Understanding
the antecedents of parents’ adherence to FDA warnings, such as a
history of safe experiences with the risk-causing agent and adequate
knowledge of the FDA warning, is important to determine how to enhance
adherence in the least compliant population segment via
communication. The present findings indicate that such communication
efforts should target households with many young children (and hence
experienced parents). Currently, the FDA issues warnings on its
Medwatch system, which does not target specific populations (FDA
Division of Drug Information, Center for Drug Evaluation and Research,
personal communication, August 13, 2008). Our data argue for a
different approach: The FDA may benefit from issuing such a general
warning regarding OTC-CCM, but it may benefit further by issuing a
warning targeting the parent group least likely to follow its
recommendation, namely, experienced parents. Moreover, it may help to
disseminate the warning via packaging information rather than public
announcement. Also, to counteract the underweighing of rare events in
experienced parents, it may be effective for the FDA to exploit the
affect heuristic (Slovic et al., 2002) and target the experiential
rather than the rational system (Epstein et al., 1994; Lowewenstein et
al., 2001; Slovic et al., 2004). For example, descriptions of vivid
case studies illustrating the detrimental effects of OTC-CCM may be
more effective in promoting adherence than disseminating statistical
information on prevalence of such effects.
Other potential solutions are regulatory in nature. In the US, the FDA
decides whether a medicine is OTC, behind the counter or by
prescription only. Having medications behind the pharmacy counter
(e.g., Pseudoephedrine) or by prescription only reduces the risk of
counterindicated use at the cost of availability. As the FDA makes
this decision by weighing a medication’s risks and benefits, our
results suggest an additional risk regarding OTC-CCM-poorer adherence
to the warning among experienced parents. While the benefits of
making these medications widely available may still outweigh the risk
of lower adherence, a careful analysis seems warranted. Follow-up
studies should identify the ideal quantity of information and mode of
communication to optimize adherence.
References
Akhavan-Toyserkani, G., Chang, Y. J., & Ahmad, S. R. (2007, September
17). Postmarketing safety review of serious adverse events in
children less than 6 years of age associated with the use of cough and
cold medications. United States Food and Drug Administration, Division
of Drug Risk Evaluation. Retrieved from
http://www.fda.gov/ohrms/dockets/ac/07/briefing/2007–4323b1–02-FDA.pdf
Barron, G., & Erev, I. (2003). Small feedback-based decisions and their
limited correspondence to description-based decisions. Journal
of Behavioral Decision Making, 16, 215–233.
Barron, G., Leider, S., & Stack, J. (2008). The effect of safe
experience on a warnings’ impact: Sex, drugs, and
rock-n-roll. Organizational Behavior and Human Decision
Processes, 106, 125–142.
Barron, G., & Yechiam, E. (2009). The coexistence of overestimation and
underweighting of rare events and the contingent recency effect,
Judgment and Decision Making, 4, 447–460.
Birnbaum, M. H. (2004). Human research and data collection via the
Internet. Annual Review of Psychology, 55, 803–832.
Brown, D. (2008, October 7). Curbing cough syrup for kids: FDA weighs
limits on use of remedies. The Washington Post, p. HE01.
Camilleri, A., & Newell, B. (2009). The role of representation in
experience-based choice. Judgment and Decision Making, 4,
518–529.
Cassidy, R. E. (2006). Children’s health and the social
theory of risk: Insights from the British measles, mumps, and rubella
(MMR) controversy. Social Science & Medicine, 65, 1059–1070.
Cassidy, R., Cresswell, T., Wilson, D., & Panter-Brick, C. (2006). A
survey of UK parental attitudes to the MMR vaccine and trust in medical
authority. Vaccine, 24, 177–184.
Centers for Disease Control and Prevention (CDC). (2007). Infant deaths
associated with cough and cold medications — two states, 2005.
MMWR. Morbidity and Mortality Weekly Report, 56, 1–4.
Retrieved from
http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5601a1. htm
Epstein, S. (1994). Integration of the cognitive and the psychodynamic
unconscious. American Psychologist, 49, 709–724.
Erev, I., & Barron, G. (2005). On adaptation, maximization, and
reinforcement learning cognitive strategies. Psychological
Review, 112, 912–931.
Fox, C., & Hadar, L. (2006). Decisions from experience sampling error
prospect theory: Reconsidering Hertwig, Barron, Weber & Erev (2004).
Judgment and Decision Making, 1, 159–161.
Fox, C., & Hadar, L. (2009). Information asymmetry in decision from
description versus decision from experience. Judgment and
Decision Making, 4, 317–325.
Glasziou, P.P. (2002). Analgesia and public health: What are the
challenges? American Journal of Therapeutics, 9, 207–213.
Hertwig, R., Barron, G., Weber, E., & Erev, I. (2004). Decisions from
experience and the effect of rare events in risky choices.
Psychological Science, 15, 534–539.
Hobson-West, P. (2007). Trusting blindly can be the biggest risk of all:
Organized resistance to childhood vaccination in the UK.
Sociology of Health & Illness, 29, 198–215.
Kahneman, D., & Tversky, A. (1979). Prospect theory: An analysis of
decision under risk. Econometrica, 47, 263–291.
Li, S. Y., Rakow, T., & Newell, B. R. (2009). Personal experience in
doctor and patient decision making: From psychology to medicine.
Journal of Evaluation in Clinical Practice, 15, 993–995.
Loewenstein, G. F.,Weber, E. U., Hsee, C. K., & Welch, E. S. (2001).
Risk as feelings. Psychological Bulletin, 127, 267–286.
Morgan, M., Fischhoff, B., Bostrom, A., & Atman, C. (2002).
Risk communication: A mental models approach. New York:
Cambridge University Press.
National Public Radio/Kaiser Family Foundation/Harvard School of Public
Health. (2007). Children’s OTC cold medicines:
The public, and parents weigh in. Retrieved from
http://www.kff.org/kaiserpolls/pomr121307pkg.cfm
Roumie, C. L., & Griffin, M. R. (2004). Over-the-counter analgesics in
older adults. Drugs & Aging, 21, 485–498.
Schaefer, M., Shehab, N., Cohen, A. L., & Budnitz, D. S. (2008).
Adverse events from cough and cold medications in children.
Pediatrics, 121, 783–787.
Schwarz, N. (2007). Cognitive aspects of survey methodology.
Applied Cognitive Psychology, 21, 277–87.
Slovic, P., Finucane, M., Peters, E., & MacGregor, D. G. (2002). The
affect heuristic. In T. Gilovich, D. Griffin, & D. Kahnemann (Eds.)
Heuristics and biases: The psychology of intuitive judgment
(pp. 397–420). New York: Cambridge University Press.
Slovic, P., Finucane, M., Peters, E., & MacGregor, D. G. (2004). Risk
as analysis and risk as feelings: Some thoughts about affect, reason,
risk, and rationality. Risk Analysis, 24, 311–322.
United States Census Bureau. (2001). Population change and distribution
1990 to 2000. Census 2000 Brief, C2KBR/01–2. Retrieved from
http://www.census.gov/prod/2001pubs/c2kbr01–2.pdf
United States Food and Drug Administration (FDA). (2008). Public
health advisory: FDA recommends that over-the-counter (OTC) cough and
cold products not be used for infants and children under 2 years of
age. Retrieved from
http://www.fda.gov/Drugs/DrugSafety/
PublicHealthAdvisories/ucm051137.html
This document was translated from LATEX by
HEVEA.

