Inquiry Project: Intermolecular Attraction and Boiling Points of Straight Chain Amides
I. Introduction:
This purpose of this project is to examine the relative importance of hydrogen bonding and the C=O dipole-dipole interactions in amides. The boiling/melting points of primary, secondary, and tertiary amides will be compared to determine the relationship between the variation of the structure and boiling/melting point. Also, the boiling/melting points of various amides with the same mass will be compared to help evaluate the relationship between the intermolecular forces and the mass. Is the mass or the structure more important in terms of intermolecular forces? What is the trend for the boiling and melting points of amides and why do the compounds follow such a trend? These are some of the questions investigated and answered in this inquiry project.
II. Discussion:
When a carboxylic acid reacts with an amine an ammonium carboxylate salt is formed. When this salt is heated, to a temperature over 100oC, an amide is formed. An amide has a carbonyl group as well as a nonbonding pair of electrons on the nitrogen atom. An amide may be primary, a single alkyl group attached to the nitrogen along with two hydrogens; it has a structure of R-CO-NH2. (See Figure 1.) The amide may also be secondary, with one other alkyl group attached to the nitrogen; the structural formula is R-CO-NHR’. (See Figure 2.) The third type of amide is the tertiary amide. This has two other alkyl groups attached to the nitrogen; its structure is R-CO-NR’2. (See Figure 3.) The amide functional group is considered to be neutral. The amides are only weakly basic and a concentrated strong acid is required to protonate an amide and then the protonation occurs on the carbonyl oxygen rather than the nitrogen.
This acid derivative
has a highly polar carbonyl group. The carbonyl group has a dipole moment
of ~0.86 D. This dipole leaves the
oxygen with a partial negative charge and the carbon with a partial positive
charge. Also, the amide has a resonance picture that indicates it is strongly
polar in nature. (See Figure 4.) The partial positive of one molecule
will orient
itself
next to the partial negative of another molecule in the more stable negative-positive
arrangement. This arrangement results in a dipole-dipole force that is
a strong attractive intermolecular force. Of all acid derivatives, amides
have the highest boiling points. This is not only due to the dipole-dipole
interaction; primary and secondary amides also experience hydrogen bonding.
(See
Figure 5.) The strength of the hydrogen bonding is stronger than the
dipole-dipole interaction, so it is expected that primary and secondary
amides have higher boiling/melting points than tertiary amides. Tertiary
amides lack the N-H bonds and cannot participate in hydrogen bonding; however,
they are good hydrogen bond acceptors.
III. Data:
|
|
|
|
|
45.0408 g/mol; BP 210° C |
59.0676 g/mol; BP 198° C |
73.09 g/mol; BP 153 |
|
59.0676 g/mol; BP 221° C |
73.09 g/mol; BP 204-206 |
87.1212 g/mol; BP 166° C |
|
73.09 g/mol; BP 213° C |
87.1212 g/mol; BP 205-206 |
101.48 g/mol; BP 174-176 MP -45° C |
|
87.1212 g/mol; BP 216oC |
101.48 g/mol; MP –5.2° C BP 156° C at 90 torr |
115.87 g/mol; BP 80° C MP -40° C |
|
101.48 g/mol; MP 115° C |
115.87 g/mol; BP 182-186° C MP –25.5° C |
|
|
115.87 g/mol; MP 101-104 |
The letters in parentheses before the name will be the letter by which the compounds are referred in this project write-up. |
First, examining the series of amides across the rows of the data table allows for a comparison of primary, secondary, and tertiary amides with the same number of carbons attached to the carbonyl group while varying the groups attached to the nitrogen. These compounds do not have the same molar mass, but an obvious trend is observed.
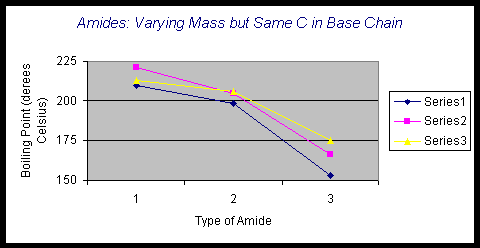 In
the graph:
In
the graph:
Series 1 = compounds A, B, and C
Series 2 = compounds D, E, and F
Series 3 = compounds G, H, and I
In each series, the primary amide has the highest boiling point of the three, followed by the secondary amide and, finally, the tertiary amide. The 1o amide has two N-H bonds and hydrogen bonding may occur at two sites on these types of amides. The 2o amide has only one N-H bond and only one site of hydrogen bonding. The 3o amide has no N-H bonds and does not participate in hydrogen bonding; it only has the dipole-dipole interaction brought about by the carbonyl group. The 1o amide of each series has the smallest mass, but it also has the most hydrogen bonding and the greatest amount of intermolecular forces associated with the molecules; it makes sense that the 1o amides have the highest boiling points within the series. The 3o amides of each series have the greatest mass but, with only dipole-dipole forces, have the smallest intermolecular forces and therefore, the lowest boiling points. Although the general trend of organic compounds is that the greater mass compounds generally have the higher boiling points, this is because the greater mass usually has greater intermolecular forces. This is not the case with the amides; the higher mass compound in a series has less intermolecular force and a lower boiling point. The intermolecular forces are more significant than the mass in terms of boiling points of amides. Also, within a series, there is a small difference between the boiling points of a 1o and a 2o amide. The difference between the boiling points of the 2o and the 3o is much larger. This is due to the fact that the 1o and the 2o amides have hydrogen bonding, while the 3o amide does not have hydrogen bonding
Second, examining the series of compounds that have the same mass arranged in a different manner, allows for a comparison of the amides, with mass being removed from the discussion. This corresponds to the diagonal lines in the data table.
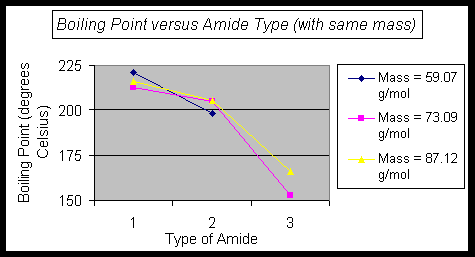
In the Graph:
Mass = 59.07 g/mol
Compound = D & B
Mass = 73.09 g/mol
Compound = G, E, & C
Mass = 87.12 g/mol
Compound = J, H, & F
Also, in these series, the primary amide has the highest boiling point, followed by the secondary, and then the tertiary amide. A similar trend may be seen with the melting points of the amides that did not have boiling points available.
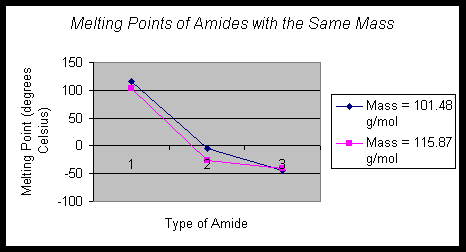
In the graph:
Mass = 101.48 g/mol
Compound = M, K, & I
Mass = 115.87 g/mol
Compound = O, N, & L
The mass of the
compounds in each series is the same; the only difference is the arrangement
of the groups. Again, the boiling and melting points of the 1o
amides are higher than that of the 3o amides due to the strength
of the hydrogen bonding available to the 1o and 2o
amides. Mass does not appear to make as significant affect on the boiling
or melting points of amides as does the type of amide.
There is also the dipole-dipole interaction caused by the C=O group, that creates a partial positive charge on the carbon and a partial negative charge on the oxygen. This carbonyl group does contribute to the boiling points of the amides. In the 1o amides, the hydrogens attached to the nitrogen allow the carbonyl group more exposed and able to more easily interact with the carbonyl group of other molecules, thus giving more dipole-dipole attractions. The two alkyl groups attached to the nitrogen in the 2o amide do not allow the carbonyl group to be as exposed and most likely decrease its ability to form those dipole-dipole interactions. This probably lends, to a small extent, to the lower boiling points of 3o amides.
V. Conclusion:
Mass, usually a significant piece in explaining boiling point trends, is not as important to the boiling point trend of amides as the type of amide, primary, secondary, or tertiary. The greater the number of hydrogens attached to the nitrogen, the greater the strength of the hydrogen bonding and therefore the greater the boiling points of the amides. This is the opposite trend expected when examining mass. The hydrogen bond being stronger than the dipole-dipole interactions caused by the carbonyl group give the hydrogen bonding the greater importance in the boiling point of the amide. Although, the somewhat high boiling points of the 3o amides, do indicate that the dipole-dipole interactions are significant when comparing amides to other types of compounds with similar molar masses.
VI. Bibliography:
Online sources:
Beilstein’s
Crossfire Data Base
ChemExper
Chemical Directory maintained by the Universite de Lausanne at www.chemexper.com/ccd/power.index.shtml
Chem Finder
at www.chemfinder.com
Print sources:
Organic Chemistry:
A Short Course, 10th edition, by Hart, Craine, and Hart
Organic Chemistry,
4th edition by L. G. Wade, Jr.