OFFICE OF NAVAL RESEARCH
END-OF-THE-YEAR REPORT
PUBLICATIONS/PATENTS/PRESENTATIONS/HONORS/STUDENTS REPORT
for
GRANT: N00014-92-J-1369
PR No: 96PR02211-00
Title of GRANT:
"Polyaniline and Polypyrrole: Investigation of the Relationship Between Molecular Conformation and Electronic and Related Properties and Application to Novel Liquid Crystal Display Devices" (01 June 1997 - 31 December 1997)
"Mesomolecular Anilines:
New Horizons in Electrically Conducting Organic Materials" (01 January 1998 - 31 May 1998)
Name of Principal Investigator:
Alan G. MacDiarmid
Name of Organization:
Address of Organization:
231 South 34th Street
Philadelphia, PA 19104-6323
Date Submitted:
Reproduction in whole, or in part, is permitted for any purpose of the United States Government.
This document has been approved for public release and sale, its distribution is unlimited.
![]()
Office of Naval Research
Publications/Patents/Presentations/Honors Report
|
PR Number: |
96PR02211-00 |
|
Grant Number: |
N00014-92-J-1369 |
|
Grant Title: |
"Mesomolecular Anilines: New Horizons in Electrically Conducting Organic Materials" |
|
Principal Investigator: |
Alan G. MacDiarmid |
|
Mailing Address: |
University of Pennsylvania, Department of Chemistry, 231 South 34th Street, Philadelphia, PA 19104-6323 |
|
Phone Number: |
215-898-8307 |
|
Fax Number: |
215-898-8378 |
|
E-mail Address: |
|
|
http address: |
![]()
Part I
h. Invited Presentations
|
June 2 1997 |
ONR Polymer Program Review Mtg, Jacksonville, FL |
|
|
June 14-22 1997 |
Conference: "Solid State Physics," Katsyveli, Ukraine |
|
|
June 23-28 1997 |
Conference: "Towards Molecular Electronics," Poznan, Poland |
|
|
July 11-12 1997 |
Materials for Electronics & Imaging Conf., Univ. of Rochester, NY |
|
|
September 1-5 1997 |
"Polymers for Advanced Technologies [PAT `97]," Leipzig, Germany |
|
|
September 8-13 1997 |
ACS National Mtg., Las Vegas, NV |
|
|
September 19 1997 |
Seminar Series, Ohio State Univ., Columbus, OH |
|
|
October 14 1997 |
Seminar Series, Carnegie Mellon Univ., Pittsburgh, PA |
|
|
October 17 1997 |
Center for Photochemical Sciences, Plenary Lecture, Bowling Green, OH |
|
|
November 7 1997 |
HIDE/DARPA "Kickoff Mtg., Rosslyn, VA |
|
|
December 1-3 1997 |
MRS National Mtg., Boston, MA |
|
|
December 15-18 1997 |
TRP Final Review, Malibu, CA |
|
|
January 28-30 1998 |
SPIE Photonics West 1998, San Jose, CA |
|
|
March 5 1998 |
2nd Annual Review Mtg. ARO-MURI Program, Aberdeen Proving Grounds, MD |
|
|
March 28-31 1998 |
ACS National Mtg., Dallas, TX |
|
|
April 28-30 1998 |
Society of Plastics Engineers (ANTEC `98), Atlanta, GA |
|
|
May 29 - June 2 1998 |
ONR Polymer Program Review, Jacksonville, FL |
|
|
i. Submitted Presentations |
||
|
March 16-18 1998 |
American Physical Society Mtg, Kansas City MO |
|
j. Honors/Awards/Prizes for contract grant employees
k. Full-time graduate and post-doctoral associates supported under this R & T project
Graduate Students
J. Feng
Z. Huang
P-C. Wang
Post-Doctoral Associates
N o n e
![]()
Part II
a. Principal Investigator: Alan G. MacDiarmid
b. Current Telephone Number: 215-898-8307
c. Cognizant ONR Program Officer: Kenneth J. Wynne
d. Program Objective:
The primary, closely inter-related, objectives are:
We have:
The results described above have already had, and will continue to have, a constantly increasing impact on the use of thin films (semi-transparent if needed) of conducting polymers as substitutes for gold electrodes in electrostrictive/piezoelectric devices, for electro-acoustic applications and in liquid crystal display technology. The hitherto unnoticed enormous potential role of isomers in polyaniline promise to provide a method of increasing the conductivity of polyaniline, probably the most technologically important conducting polymer today, by several orders of magnitude. The completely unexpected, excellent properties of (aniline)8 as an inexpensive, rugged, sensitive, reversible sensor for VOCs is already having an impact in DOD objectives for the 21st century.
Students Currently Working On The Project
Graduate Students
Jing Feng
Pen-Cheng Wang
Post-Doctoral Fellows
N o n e
![]()
Part III
Explanatory Text For 5-part View Graph
Note:
Previous Grant
Objective
The primary objective was to determine optimum conditions for controlled deposition of thin films of conducting polymers and oligomers on substrates and their evaluation for specific technological applications. This involved optimization of the newly discovered phenomenon described in its preliminary form in our report of July 1, 1995. We showed that the electronic properties, conductivity, molecular conformation, and adhesion properties of thin films of the conducting polymers, polypyrrole and polyaniline, when deposited from dilute aqueous polymerizing solutions of the monomer, by our in-situ "1-dip" process are greatly dependent on the degree of hydrophilicity/hydrophobicity of the substrate.
Results
Studies conducted in the present report have concentrated primarily on ascertaining how the nature of in-situ-deposited polypyrrole changed with increasing film thickness. Obviously, as soon as the first monolayer of polypyrrole had covered the surface of the original hydrophobic or hydrophilic substrate, the degree of hydrophobicity/hydrophilicity for successive layers had been changed since successive layers were now formed on a surface having a different hydrophobicity/hydrophilicity from the original substrate surface. As can be seen from the figure:
It is not yet entirely clear how or why the "memory effect" occurs. However, it is self-evident that this effect is most important when constructing polymer electrodes for use in electrical/electronic devices.
We have deposited polypyrrole films on polyurethane films in collaborative studies with Prof. Q. Zhang (Materials Research Lab., Penn State Univ., ONR Grant N00014-96-1-0418) for projected use in electrostrictive systems for electro-acoustic applications. The relatively elastic polypyrrole film electrode as compared to the conventionally used "non-elastic" gold film electrode is envisaged as providing significantly improved electro-acoustic applications.
Impact
The preliminary studies are extremely encouraging as shown, for example, in:
(1) "Preparation and Characterization of Electrostrictive Polyurethane Films with Conductive Polymer Electrodes,"
Polym. Adv. Technol., 9, (1998) 317-321.
(2) Preliminary patent, "Polymeric Electrostrictive Systems," Filed Oct. 3, 1997.
The above manuscript/patent compares the electric field induced strain coefficient, R, of the polypyrrole-electroded and gold-electroded solution-cast polyurethane films with thicknesses of 0.1 mm and 0.2 mm, respectively, as a function of frequency. The remarkable similarity is clearly apparent!
As shown in the figure, preliminary collaborative studies with Prof. Zhang have been extended during the present report period to the new piezoelectric copolymer of poly(vinylidene fluoride-trifluoroethylene). These ongoing studies are continuing and strongly suggest that conducting polymer electrodes may be extremely useful as a substitute for evaporated gold electrodes in electro-acoustic applications in this new system.
New Grant
Objective
The main thrust of the proposed research for the new grant focuses on oligomeric and mesomolecular anilines. The whole field of electroactive polymers has undoubtedly been hindered by the absence of information concerning mesomolecular species - species larger than small oligomers but smaller than polymers - species in which the band structure of the polymer has almost been attained. The band structure of polyacetylene, for example, is closely approached after as small as 20 conjugated linkages. As has been shown to be the case in the thiophenes, a better understanding of mesomolecular anilines will undoubtedly lead to a better understanding of the polyaniline field resulting in increased conductivity and improved mechanical properties. Polyaniline is presently the leading conducting polymer in technology, yet it is highly likely that its true intrinsic conductivity is far below that of what has been observed experimentally to date. The present research will focus primarily on both fundamental scientific issues as well as possible technologically important properties of these materials.
Careful attention will, of course, be paid to looking for unexpected phenomena or possible technological uses for these new materials.
Results
(A) Oligomeric and mesomolecular anilines may be end-capped with a combination of a variety of groups such as phenyl, amine, etc. We have been particularly interested in completely reduced oligomers of the type
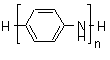
capped with a phenyl group at one end and an amine at the other, since they offer the opportunity of controllable increase in molecular weight by a completely new approach we have devised. We have found that oxidative coupling only occurs with the reduced "leucoemeraldine" oxidation state of the oligomer, it does not occur with the partly oxidized "emeraldine" oxidation state.
The tetramer, octamer and hexadecamer have been completely characterized by elemental analysis, GPC molecular weight, mass spectroscopy, vis/uv and infrared spectroscopy. Full characterization of the "32-mer" (n=32) is nearing completion.
Oligomers of Aniline
(emeraldine oxidation state)
![]()
Tetraaniline
![]()
Octaaniline
![]()
Hexadecaaniline
("16-mer")
A patent has been filed on this work: "Oligomeric Anilines and Soluble Polypyrroles as Sensors for Organic Compounds." Inventors: A.G. MacDiarmid, J. Feng and W.J. Zhang; US Patent Filing No. PCT/US97/13148, Filed July 25, 1997.
The oligomeric and mesomolecular anilines are only useful in elucidating the nature of polyaniline if they are in the same oxidation state as the polyaniline with which they are being compared. The important (dopable) oxidation state of polyaniline is the half oxidized emeraldine oxidation state. Unfortunately, all too many otherwise excellent physical studies have sometimes been performed on materials where oxidation state is uncertain. In our ongoing studies we have taken extreme and painstaking care in converting our higher molecular weight anilines to the exact emeraldine oxidation state (by a procedure which is too lengthy to explain in this summary). Their properties can now be readily compared with those of polyaniline in this exact emeraldine state.
We have shown by 1H and 13C NMR studies in d6-DMSO solution that the aniline tetramer exists in the rapidly interconverting isomeric forms given in the figure. In principle, each of these two forms could also exist in two different isomeric cis or trans isomeric forms (see figure) for which we are now searching. The number of possible different isomeric forms increases enormously as the size of the molecule increases. The extremely large number of possible co-existing isomeric forms in polyaniline may account for its very much smaller conductivity than expected. An increased understanding of factors favoring a given isomer will hopefully lead to the controlled synthesis of a given, desired isomeric form of polyaniline, thus resulting in achievement of its intrinsic (high) conductivity and improved electronic, optical and mechanical properties in general.
At the same time that the oligomeric and mesomolecular syntheses were being performed (supported by this grant) we were investigating the use of conducting polymers as sensors for volatile organic compounds on a Technology Reinvestment Program Contract (No. N00014-95-2-008) subcontracted from Hughes Research Laboratories and Abtech Scientific, Inc. As a natural extension of the oligomer work, the use of octaaniline in a sensor was investigated. This sensor work was therefore funded in part by both the present ONR grant as well as by the TRP program contract referenced above.
This work has proceeded unexpectedly well and two of the eight sensors used in a demonstration array (detection limits ~30 ppm) for DOD at Hughes' Malibu Laboratory at the final meeting of the Consortium contained octaaniline sensors!
A patent on this sensor work was filed on July 25, 1997: "Oligomeric Anilines and Soluble Polypyrroles as Sensors for Organic Compounds." Inventors: A.G. MacDiarmid, J. Feng and W.J. Zhang; US Patent Filing No. PCT/US97/13148.
We have been especially interested in sensors which give a rapid and reversible response to aliphatic and aromatic hydrocarbons. We found that the octaaniline described above, doped with dodecylbenzene sulfonic acid, is more sensitive than our earlier polypyrrole and polyaniline sensors and displayed good stability. A solution of the doped octamer in chloroform was spun on to interdigitated gold electrodes to produce a film. The sensor was held in air and then in toluene vapor and the change in resistance was measured.
The unexpectedly large sensitivity of the octaaniline sensors as compared to polyaniline sensors (which are less stable environmentally) offer many fundamental scientific challenges as well as excellent potential technological opportunities.
![]()
Supporting Text for View Graph:
"Use of a New Type of Polymerized Aniline as a Reversible Sensor for Volatile Organic Compounds (VOCs)"
At the same time that the oligomeric and mesomolecular aniline syntheses were being performed (supported in whole by this grant) we were investigating the use of conducting polymers as sensors for volatile organic compounds on a Technology Reinvestment Program Contract (No. N00014-95-2-008) subcontracted from Hughes Research Laboratories and Abtech Scientific, Inc. As a natural extension of investigating the properties of the oligomer, the use of octaaniline in a sensor was investigated. This sensor work was therefore funded by both the present ONR grant as well as by the TRP program contract referenced above.
This work has proceeded unexpectedly well and two of the eight sensors used in a demonstration array (detection limits ~30 ppm) for DOD at Hughes' Malibu Laboratory at the final meeting of the Consortium contained octaaniline sensors!
A patent on this sensor work was filed on July 25, 1997: "Oligomeric Anilines and Soluble Polypyrroles as Sensors for Organic Compounds." Inventors: A.G. MacDiarmid, J. Feng and W.J. Zhang; US Patent Filing No. PCT/US97/13148.
We have been especially interested in sensors which give a rapid and reversible response to aliphatic hydrocarbons. We found that the octaaniline described above, doped with dodecylbenzene sulfonic acid, is more sensitive than our earlier polypyrrole and polyaniline sensors and displayed good stability. A solution of the doped octamer in chloroform was spun on to interdigitated gold electrodes to produce a film. The sensor was held in air and then in toluene vapor and the change in resistance was measured.
In our collaborative studies with Hughes Research Laboratory, we, at Penn, tested new sensor materials by the rough method described above. We then sent the best sensors to Hughes Laboratories where they were then tested in a specially equipped laboratory specializing in the detection of vapors of various gases mixed with air in the ppm range.
Test times of 120 seconds were routinely used in our studies for ease of comparison of various materials. For testing purposes, toluene vapor was always used as the test analyte. Toluene, similar to most hydrocarbons, gave an increase in resistance; polar vapors such as alcohols resulted in a decrease in resistance. The sensitivity was measured as the percentage change in resistance (D R) defined as D R = [(R-R0)/ R0 x 100]% where R0 is the initial resistance of the sensor and R is the resistance after a given time period.
The octaaniline sensor was ~ 1000 times more sensitive than a polyaniline sensor (both doped by dodecylbenzene sulfonic acid) when exposed to air saturated with toluene vapor (~ 35,000 ppm) under the same experimental conditions. Thus the polyaniline gave a 35% change in resistance while the octaaniline sensor gave a 30,000% change in resistance! Moreover, the polyaniline sensor when re-exposed to air was only partly reversible (86%), while the octaaniline sensor was completely reversible (99.7%) within experimental error.
Since the expiration of our TRP contract mentioned above, we have been continuing our sensor studies which presently are supported solely by this grant.
We find by refining the octaaniline sensor that it is by far the most sensitive sensor we have investigated. It can easily and readily (and reversibly) detect ~ 35 ppm of toluene vapor admixed with air (see figure). Resistance changes were measured by a simple, inexpensive, undergraduate teaching laboratory ohmmeter. As is readily apparent from the data points, this sensitivity can readily (and is constantly being) increased.
We hypothesize that the resistance changes are the result of analyte vapor-induced changes in the relative amounts of differing isomeric species in the tetramer.
The unexpectedly large sensitivity of the octaaniline sensors as compared to polyaniline sensors (which are less stable environmentally) offer many fundamental scientific challenges as well as excellent potential technological opportunities.
![]()
Supporting Text for View Graph:
In-Situ
Deposited Thin Films of PolypyrroleThe primary objective was to determine optimum conditions for controlled deposition of thin films of conducting polymers and oligomers on substrates and their evaluation for specific technological applications. This involved optimization of the newly discovered phenomenon described in its preliminary form in our report of July 1, 1995. We showed that the electronic properties, conductivity, molecular conformation, and adhesion properties of thin films of the conducting polymers, polypyrrole and polyaniline, when deposited from dilute aqueous polymerizing solutions of the monomer, by our in-situ "1-dip" process are greatly dependent on the degree of hydrophilicity/hydrophobicity of the substrate.
Studies conducted in the present report have concentrated primarily on ascertaining how the nature of in-situ-deposited polypyrrole changed with increasing film thickness. Obviously, as soon as the first monolayer of polypyrrole had covered the surface of the original hydrophobic or hydrophilic substrate, the degree of hydrophobicity/hydrophilicity for successive layers had been changed since successive layers were now formed on a surface having a different hydrophobicity/hydrophilicity from the original substrate surface. As can be seen from the figure:
It is not yet entirely clear how or why the "memory effect" occurs. However, it is self-evident that this effect is most important when constructing polymer electrodes for use in electrical/electronic devices.
We have deposited polypyrrole films on polyurethane films in collaborative studies with Prof. Q. Zhang (Materials Research Lab., Penn State Univ., ONR Grant N00014-96-1-0418) for projected use in electrostrictive systems for electro-acoustic applications. The relatively elastic polypyrrole film electrode as compared to the conventionally used "non-elastic" gold film electrode is envisaged as providing significantly improved electro-acoustic applications.
The preliminary studies are extremely encouraging as shown, for example, in:
(1) "Preparation and Characterization of Electrostrictive Polyurethane Films with Conductive Polymer Electrodes,"
Polym. Adv. Technol., 9, (1998) 317-321.
(2) Preliminary patent, "Polymeric Electrostrictive Systems," Filed Oct. 3, 1997.
The above manuscript/patent compares the electric field induced strain coefficient, R, of the polypyrrole-electroded and gold-electroded solution-cast polyurethane films with thicknesses of 0.1 mm and 0.2 mm, respectively, as a function of frequency. The remarkable similarity is clearly apparent!
As shown in the figure, preliminary collaborative studies with Prof. Zhang have been extended during the present report period to the new piezoelectric copolymer of poly(vinylidene fluoride-trifluoroethylene). These ongoing studies are continuing and strongly suggest that conducting polymer electrodes may be extremely useful as a substitute for evaporated gold electrodes in electro-acoustic applications in this new system.