Ethane
C2H6 can rotate
rather freely being a smaller compound.
The sigma bond can overlap while the ends of the molecule
rotate; the
two methyl groups are not held in fixed positions relative to
one another. The different spatial
arrangements formed by these rotations about a single bond are called
conformations or conformers. We rotate
the molecule bond by degrees from 0-360.
There
are an infinite number of conformations about any sigma bond so we
focus on the
most significant; eclipsed and staggered conformations.
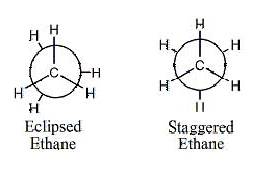
Staggered conformations are almost always
favored. In an eclipsed conformation the
carbons are aligned so that the hydrogens are lined up with each other. This creates steric hindrance between
them. In a staggered conformation the
atoms are all equally spaced from each other.
Ethane being rather small and only having simple hydrogens to
rotate has
only two major conformations. The
staggered and eclipsed conformations repeat every 120 degrees.
The
eclipsed conformation of ethane is less stable than the staggered
conformation. Energies for
these
rotations are measured using Chem 3D,
drawing an ethane molecule and minimizing its energy will start
it in
the staggered conformation. The
staggered conformation is the most stable of all possible conformations
of
ethane, since the angles between C-H bonds on the front and rear
carbons are
maximized which minimizes the energy.
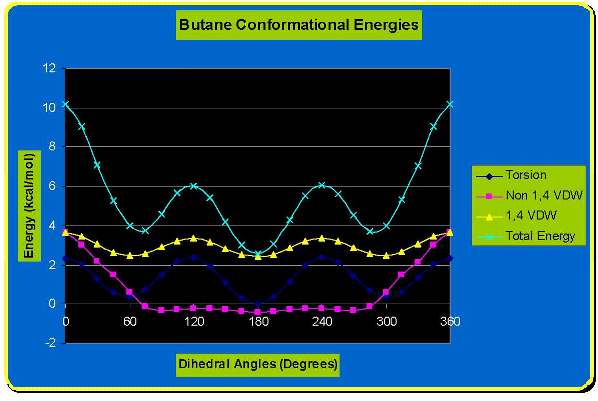
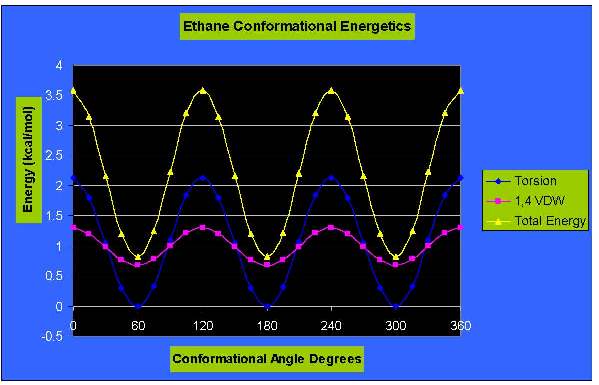
The Total Energy is visualized on the graph by the green curve. The minimums can be seen on the graph at 60,
180 and 300 degrees. In the eclipsed
form, the electron densities on the C-H bonds are closer together than
they are
in the staggered form. When two C-H bonds are brought into a dihedral
angle of
zero degrees, their electron clouds experience repulsion, which raises
the
energy of the molecule. The eclipsed conformation of ethane has three
such C-H
eclipsing interactions, they can be seen on the graph at 0/360, 120, 300 degrees.


Eclipsing
interactions are an example of a general phenomenon called steric
hindrance,
which occurs whenever bulky portions of a molecule repel other
molecules or
other parts of the same molecule. The steric hindrance causes
resistance to
rotation, also called torsional strain.
This strain was also measured by the Chem 3D program and is
visualized
in blue on the graph. As shown on the
graph the maximums and minimums also correspond to the eclipsed and
staggered
conformations as before. The red curve
is the measurement of the 1,4 Van der Waals forces; which again
correspond to
the eclipsed and staggered conformations.
These
two forms of the ethane molecule do not exist independently of each
other; the molecule ist in constant motion. The eclipsed and staggered conformation are
not considered isomers because of their rapid interconversion.
Butane,
C4 H10, has more conformations than ethane since it has four carbon
atoms
and its dihedral angles could vary
across three C-C bonds. We focus on the central C2 -- C3
bond and treat the end carbons generally as
methyl groups. Conformationally, butane is more complex than ethane because
instead of rotating alike hydrogens as in ethane, butane rotates two
hydrogrens
and one methyl group. The methyl group
creates more steric hindrance especially as they approach each other. We analyze the conformational energies as we
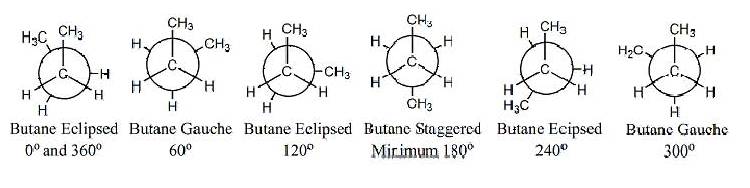
did ethanes’. Butane will have four
conformations of importance; two different eclipsed conformations and
two
different staggered conformations. These
conformations differ by the relative positions of the two methyl
substituents.
The butane molecule is drawn in Chem 3D and energy minimized. This places the molecule at its optimal
energy which corresponds to the staggered conformation where the two
methyl
groups are furthest from each other.
This is visualized at 180 dergrees on the graph and is the most
stable
conformation. This particular staggered
conformation is called anti. The other staggered conformation has the
methyl
groups at a dihedral angle of 60 and 300
degrees on the graph. This is called the gauche conformation. The
gauche form
is less stable than the anti form due to
steric hindrance between the two methyl groups but still is more stable
than
the eclipsed formations. Such an
interaction is often referred to as a gauche-butane interaction because
butane
is the first alkane discovered to exhibit such an effect.
The eclipsed formations have two energy
levels as well. At the 0 or 360 angle of
rotation the steric energy is at its maximum and is the least favorable
conformation. In this conformation the
methyl groups are overlapped with one another.
The other eclipsed conformation occurs at the angles of 60 and
300
degrees. In these conformations the
methyl groups are eclipsed by single hydrogens and thus have less
steric
interaction. However it is still less favorable than either of the
staggered
conformations. The Newman Projections
which illustrate these interaction by sighting directly down the C2 --
C3 bond showing the conformations
discussed. The graph illustrates the
total energy in the purple curve and the torsion (blue curve), non 1,4
Van der
Waals (red curve), 1,4 Van der Waals (green curve) separately as well.