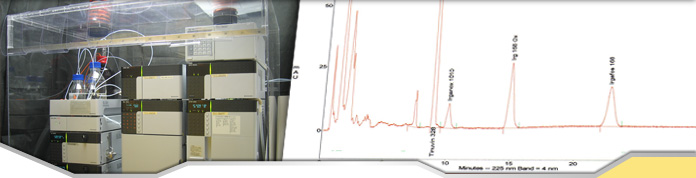
XRF

 Mosley also created the first attempted X-ray
spectrometer but it proved to be inefficient since it used electrons
that were lost through heat for its energy source. The Bragg brothers also developed an x-ray spectrometer with
the use of a slit and collminator but could not solve the efficiently
problem. An XRF spectrometer was developed by Friedman and Birks
in 1948, their device was built with a diffractometer and used a Geiger
counter for detection of the x-rays. This method proved to be
very sensitive for most of the atomic number range. Karl Manne
Georg Siegbahn of Sweden who won the Nobel Prize in 1924 (pictured
right). He used electrons of high energy for excitation and
measured the x-ray wavelengths of the elements. Siegbahn's work
to improve air pumps and x-ray tubes increased the radiation
intensity.He continually increased the accuracy of his measurements
through crystal gratings and spectographs. These studies lead to
the discovery of the x-radiations of elements. The precision of
his instruments and measurements lead to the complete documentation of
the energy and radiation conditions in the electron shells of
atoms. To read a complete history of all his work please visit http://nobelprize.org/nobel_prizes/physics/laureates/1924/siegbahn-bio.html
Mosley also created the first attempted X-ray
spectrometer but it proved to be inefficient since it used electrons
that were lost through heat for its energy source. The Bragg brothers also developed an x-ray spectrometer with
the use of a slit and collminator but could not solve the efficiently
problem. An XRF spectrometer was developed by Friedman and Birks
in 1948, their device was built with a diffractometer and used a Geiger
counter for detection of the x-rays. This method proved to be
very sensitive for most of the atomic number range. Karl Manne
Georg Siegbahn of Sweden who won the Nobel Prize in 1924 (pictured
right). He used electrons of high energy for excitation and
measured the x-ray wavelengths of the elements. Siegbahn's work
to improve air pumps and x-ray tubes increased the radiation
intensity.He continually increased the accuracy of his measurements
through crystal gratings and spectographs. These studies lead to
the discovery of the x-radiations of elements. The precision of
his instruments and measurements lead to the complete documentation of
the energy and radiation conditions in the electron shells of
atoms. To read a complete history of all his work please visit http://nobelprize.org/nobel_prizes/physics/laureates/1924/siegbahn-bio.html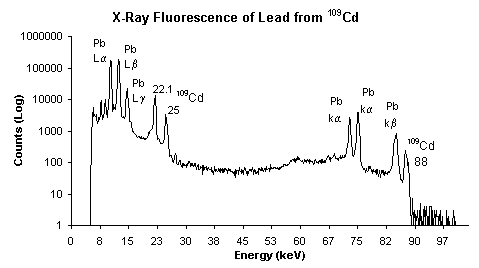 X-Ray
Fluorescence is initiated by the bombarding a
material with high energy x-rays or gamma rays and exciting the
electrons of the material. XRF is primarily used for chemical and
elemental analysis and is efficient in identifying metals like
lead. The
term X-ray comes the energy used
to bombard and excite the material and the term fluorescence refers to
the emission of lower energy radiation after higher energy radiation
has been absorbed.
X-Ray
Fluorescence is initiated by the bombarding a
material with high energy x-rays or gamma rays and exciting the
electrons of the material. XRF is primarily used for chemical and
elemental analysis and is efficient in identifying metals like
lead. The
term X-ray comes the energy used
to bombard and excite the material and the term fluorescence refers to
the emission of lower energy radiation after higher energy radiation
has been absorbed.
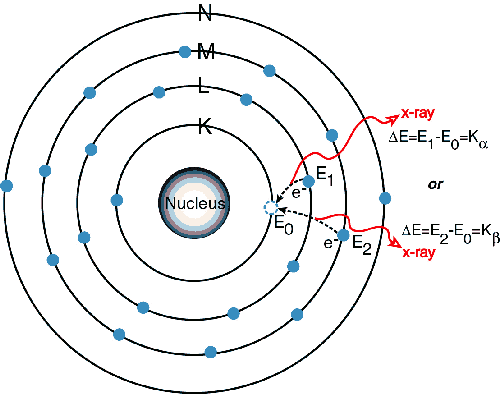
The X-rays have enough energy to
force the electron to surpass its ionization energy and actually eject
the electron from the atoms in a 'photoelectric effect', the
resulting instability forces other electrons to fall into the gaps and
thus release energy. The energy is released as a photon with a specific energy
relating to the difference in energy of the orbitals. This
emitted radiation is characteristic of the specific atoms present in
the material. The wavelength of the emitted radiation can
be calculated using Plank's Law. The inner K and L shells are most
typically involved, on the x-ray spectrum the peaks are labeled as
K,L,M,N to show the shell where the electron was originally
located.
Images from http://www.amptek.com/xrf.html.
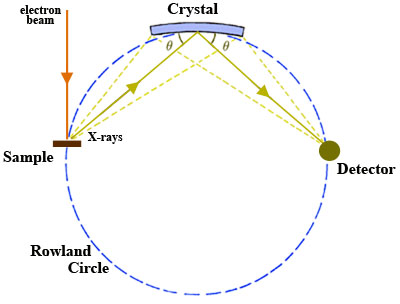
One type of XRF
spectrometer is the wavelength dispersive spectrometers that use a
diffraction crystal to focus the specific wavelengths on to the
detector. The wavelength range is adjusted by changing the angle
of
the x-rays hitting the surface of the crystal. The
x-rays are then passed from the crystal on to the solid surface
detector. The angle of the x-ray, the analytical crystal and
detector must all be contained in precise measurements of each other,
this is referred to as the Rowland Circle. The crystal is
tangent to the circle and the slit for the x-rays and the detector are
points on the circle. The circle is pictured to the left from
http://serc.carleton.edu/research_education/geochemsheets/wds.html.
One other type
of XRF Spectrometer is the Energy Dispersive spectrometer which focuses
all of the emitted x-rays directly onto an energy analyzing
detector. These are not as sensitive as its wavelength dispersive
partner and also have a lower resolution.
 Generally the XRF method
is non destructive to the subject being analyzed. The most
accurate readings are achieved when the material is ground down and
formed into a uniform sample, please refer to the Sample Preparation
page for the methods that the toys are prepared for analysis.
This extensive testing protocol is used by industry when toys are
applying for safety ratings so that their products can be marketed in
the United States and abroad. However, the XRF method is very
versatile since it operates with xray that can penetrate most any
substance. The XRF
method can be none destructive to the material and therefore is great
for
use in the field. It is also very efficient and very cost
effective. Agencies use portable XRF scanners that use
gamma rays as the energy source (Pictured to the right) to voluntarily
test toys at community events and health fairs. These XRF
scanners can also be rented rather inexpensively to test not only
children's toys but surface paints, soil, minerals and any other
household application. Accuracy has improved with these devices,
a major concern was the control of how deep the x-rays penetrated the
sample. This is an issue when testing a material for surface
paint since the substrate which may be lead free would influence and
lower the percentage results of the surface contaminants.
Generally the XRF method
is non destructive to the subject being analyzed. The most
accurate readings are achieved when the material is ground down and
formed into a uniform sample, please refer to the Sample Preparation
page for the methods that the toys are prepared for analysis.
This extensive testing protocol is used by industry when toys are
applying for safety ratings so that their products can be marketed in
the United States and abroad. However, the XRF method is very
versatile since it operates with xray that can penetrate most any
substance. The XRF
method can be none destructive to the material and therefore is great
for
use in the field. It is also very efficient and very cost
effective. Agencies use portable XRF scanners that use
gamma rays as the energy source (Pictured to the right) to voluntarily
test toys at community events and health fairs. These XRF
scanners can also be rented rather inexpensively to test not only
children's toys but surface paints, soil, minerals and any other
household application. Accuracy has improved with these devices,
a major concern was the control of how deep the x-rays penetrated the
sample. This is an issue when testing a material for surface
paint since the substrate which may be lead free would influence and
lower the percentage results of the surface contaminants.
| Spectroscopy Home |
Toy Safety Standards |
Sample Preparation |
| XRF |
AAS |
Lesson Plan |