
[ Home | Grading rubrics | Things to keep in mind ARCHIVES ]

[ Home | Grading
rubrics | Things to keep in mind ARCHIVES
]
General guidelines: pg. 77 and 78 of your lab manual
A simplified "sample paper" may be found on the 245 lecture notes website; its main function is to give you an idea of an "experimental" section in a paper.
More in-depth guidelines: The Scientific Paper.
Your general format of the paper is as follows, quoted from the Manual pg. 77:
...should summarize the work done in connection with the synthesis of 1-bromo-3-chloro-5-iodobenzene; the format for the report should be similar to that used for Articles/Notes in the Journal of Organic Chemistry. This should include:
Title
Author and affiliation
Abstract
Introduction, Results and Discussion
Experimental Section
Acknowledgements (optional)
References and footnotes should be included as needed.
***
ABSTRACT
My expectations regarding the abstract are pretty
congruous with what is presented in The Scientific Paper handout.
Quoting from pg. 5: "Many
publications require that an informative abstract accompany every paper.
For a research paper, the abstract should summarize the principle findings; ...
allow the reader to determine the nature and scope of the information given in
the paper".
I expect you:
►make brief mention of the
experimental plan & theory
►summarize findings/conclusion
►avoid citing your paper, figures, or
other papers...
Additionally, you should not be bringing up stuff that you are not going to be
mentioning in the body of your paper (no additional conclusions, insights, etc.
soaring out of the blue).
►Most of all, your abstract ought to
be "concise and self-contained"
(pg.5)!
**Grammar/proofreading/spelling COUNTS!**
INTRODUCTION
Yeah, so [intro, results & discussion] is given on one line of the lab
manual's paper instructions, but you may break it up in whatever way you feel
appropriate. If you are more comfortable making 3 separate sections, or 1,
it doesn't matter to me, as long as the content/results/analysis are all in
there somewhere!
General thoughts for your Introduction:
►WHY (aside
from being required to do so) are you writing this paper?
..in other words, what is
special about this six-step synthesis?
►Background, scope, limits?
►Mechanisms? =complete/correct/arrows/charge/equilibria
of ALL major steps...
..though, if a particular mechanism is a repeat of a previous one,
feel free to just refer back to it and discuss what players are
different this time, despite it being the same mech...
►Reasons behind running the
reactions we did?
Chemical drawing software is not required, but highly recommended for neatness/professionalism's sake. All you need to do is draw, clean-up structure, draw arrows, type captions, and then copy/paste into your word processing software. ChemDraw is available in the chemistry library computer lab. ChemSketch is available for download.
This should be a thorough intro, and I expect good, clear, and organized
writing out of you. You're not getting a final in this class, so take this
write-up seriously; it's worth TWO hour-long exams!
**I've said this already: grammar/proofreading/spelling COUNTS!**
RESULTS & DISCUSSION
Your results should be a summary of the data you got. It's OK to supply me with a nice, neat summary table of products, weights & melting ranges per crop, and % yield per step, as well as OVERALL % yield...
The discussion should tell me errors, side reactions, problems, you encountered. Spectra and analysis ought to be complete and well-explained.
►How do you *know* that you got the product you were going for? (Ex: going from the halogenated acetanilide to aniline, what peaks disappeared/appeared/remained the same in the IR and 1H NMR)? Since your Experimental section contains the full peak & spectral assignment, I don't need you to re-state Every. Single. Signal. That's time-consuming and redundant. But if it's important, please include it! (Ex: ignoring the disappearance of that strong carbonyl stretch in the IR after your amine hydrolysis would be greatly frowned upon..)
►How pure would you say you managed to make this? Did you combine a second crop, and if so, why did/didn't you?
Are there: Limitations? Implications? Comparisons to make?
Final thought: there's an unmentioned conclusion buried in here near the
end...
You don't need a separate section for it, because it should have followed from
the evidence you already presented. So, don't abruptly stop typing here,
finish up your Experimental section, and then print it and submit.
►give me some CLOSURE!
EXPERIMENTAL.
Your
experimental is kind of like an appendix or supplementary material for this
particular paper. As such, it is the caboose of the document and where I'd
go after I read your paper and wanted to reproduce your results.
I'd need to know what you did.
Model this section off the "sample paper" posted on
Dr. M's archives. Make it the repository for the
experimental procedure for each step of each substance's synthesis, as well as
for your observations (color,
shape), glassware, moles
used... Include literature values for purity (lit. melting point). It should be written for a chem-literate audience, and redundancy
and wordiness is highly discouraged.
Newsubstancething (2). 2.5 g (0.03 moles) of substance A was dissolved in 5ml methanol and added dropwise with stirring to 50 ml of a 1.5 M aqueous solution of substance B chilled to 0ºC. The resulting green solution was magnetically stirred in a 125ml erlenmeyer flask for 2 hours at room temperature until a pink solid formed and the green color dissipated. The crude solid (m.p. 179-196ºC) was collected by vacuum filtration and recrystallized in ~20 ml of 10% acetone in cyclohexane to give 2.0g (0.014 moles, 47% yield) of newsubstancething as long white crystalline rods, m.p. 201.5-203.0º (lit3 m.p. 204ºC). IR νmax (nujol mull) 3090-3049 (C-H stretch; alkene, aromatic), 1602 (C=C stretch; aromatic), [etc]. 1H NMR (CDCl3) δ(ppm) 8.1-7.8 (dt, 2H, J ~8.5Hz, 2Hz), [etc].
Reporting safety and toxicity information under each Experimental is also nice, as long as it really *is* relevant for the conditions & quantities we're working in. Don't tell me overexposure to water will cause death by asphyxiation... :D
MORE INFORMATION ON NMR.
Quoting Dr. M: "...this time I
expect the students to give a fairly complete and accurate discussion. I
have given expansion plots of the aromatic region for each compound in the
Manual which should allow identification of all the aromatic protons by chemical
shifts and coupling constants."
That's right. You've gotta report everything.
Your chemical shifts should be reported formally in the Experimental section
(should take no more than a couple lines per compound), but a detailed
discussion should be included in your results and discussion section.
Reporting 1H NMR splitting:
Include: • Peak multiplicity
• # of protons ("#H")
• Coupling constants (J, Hz) where applicable.
TERMS:
s = singlet d = doublet
t = triplet q = quartet
m = multiplet
dd = doublet of doublets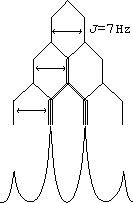
dt = doublet of triplets
Yes, there is a difference between doublet of doublets and quartet.
A quartet would be splitting by 3 identical protons, following a 1:3:3:1 pascal's triangle pattern of intensity, with equal spacing between peaks.
A doublet of doublets implies splitting by 2 non-identical protons, for example, an ortho proton with a large coupling constant and a meta proton with a small coupling constant.
References and Footnotes:
YES PLEASE! Anything that needs to be cited
(procedures, literature values, theory) ought to be cited!
That means footnotes (preferred) or end notes, or in-text citation...
I won't begrudge you HOW it is done as long as it IS done, and is complete, and
self-consistent.
Briefly citing in-text or footnote:
• Citing a particular Lab lecture should include date (Lecture, X/X/XX) or (Mallory, X/X/XX), etc.
• Citing the lab manual in text or footnote may be simply: (Manual, X), or (Mallory, X)
• G&M may be simply: (G&M, X)
• Websites should include date accessed.
FULL Citations:
The end of your paper should include the full citation for all sources that were abbreviated in your in-text / footnote / endnote citations. This includes author, title, publisher, URL, etc. Recommended style is ACS or Journal of Organic Chemistry format.
Cool stuff: **REFWORKS**
...Have lots of references? Want a program to automatically format your
sources for you so you don't have to look it up in an ACS style manual?
RefWorks is an online personal citation/bibliography
database. You need to enter the information, but it's in there, RefWorks will store, organize, and format your material on demand to match the
any journal style of your choice (ranging from JOC to IEEE, Science to
Poultry Science). You can then either print or copy/paste into your
paper.
This is a great resource which will serve you well all
through your academic career, and you can designate folders for any
subject/paper/project you choose. Once you have the information entered in
the field boxes, you're set!
NOTE: You will need to authenticate yourself with a PENN
username & password if you are using this off-campus. Additionally, you
will need to create a login name. It's all online, so you will not need to
download any software to your computer.
![]()