Eilisha Joy Bryson August
16, 2007
MISEP Chem 512 – Jacobs
Enduring Understanding Essay
Enduring
Understanding #4 –
The bonding within a
molecule determines its shape and polarity, and therefore its interactions and
reactions with other molecules. Intermolecular interactions are central to the
structure and function of the biochemical systems, and the extent and rate of
biochemical reactions govern all cellular functions. Both interactions and
reactions can be understood by analyzing energetic stability of the molecules
and bonds.
Two
types of bonds are pertinent to this Enduring Understanding. The first is due
to intramolecular forces that make
chemical covalent bonds, either polar or non-polar. Because of the octet rule
only certain arrangements of bonds will make a stable molecule, consequently
giving a molecule its geometric shape. Using the type of covalent bond, the
presence of lone pairs, and the symmetry of the shape of the molecule, you can
determine if the entire molecule
is polar or not (which is different than polar bonding). The polarity of the
molecule determines the forces occurring between it and other molecules. These intermolecular forces are basically weak bonds, but are
essential in holding molecules together. Non-polar molecules have the weakest
attractions called London forces. Polar molecules have stronger intermolecular attractions, called dipole-dipole forces.
The strongest intermolecular
force is a special type of dipole-dipole interaction called a hydrogen bond,
formed between a molecule that contains a hydrogen atom and a molecule that
contains a nitrogen, oxygen, or fluorine atom, which are highly
electronegative. Intramolecular
covalent bonds are the hardest to break and are very stable, being about 98%
stronger than intermolecular
bonds.
The covalent and intermolecular bonds discussed above result in numerous
structures and functions of biochemical systems. This is described below using
the multi-structures of proteins. The primary structure of the protein is the
long amino acid chain, and it is formed by intramolecular covalent bonds. Enzymes fold the primary
structure, creating regions of repeating patterns, a-helix
and b-sheets
being the most popular. The folds
are held together and maintain their shape due to the intermolecular force of hydrogen bonds. There can be
several differently shaped regions making up the secondary structure. The
secondary structure then folds onto itself creating a 3-dimensional shape
called the tertiary structure. This is held together and maintains its shape
due to all of the intermolecular
forces: London, dipole-dipole and hydrogen bonds, as well as ionic and
disulfide bonds which are intramolecular.
The sequence of the amino acids determines the structures of the protein and
the structures result in the proteinÕs function.
Last
summer in our Biology course, we learned about the mechanism of hormones. Dr.
WaldronÕs notes read, ÒIt begins with the binding of a hormone molecule to a
specific hormone receptor, which is a protein with a binding site which
specifically matches the shape and electrical charge distribution of the
particular hormone molecule.Ó Focusing on the hormone receptor protein, you can
see here how shape relates to function. An excellent example that we looked at
during this class was with a protein whose job was to destroy blood cells. The
proteinÕs shape, which consisted of polar and non-polar regions, allowed it to
take advantage of both the lipid and water-based properties of the cell. While
researching such proteins on the internet an article described a prion protein
that was responsible for destroying brain cells. The protein Prp takes on an
unexpected amyloid fold that consists of tight b-sheets that are difficult
to penetrate, changing it into PrPsc. These incorrect folds cause the protein
to turn brain cells into sponge-like holes. Prion is found in patientÕs cells
who had various diseases, such as Alzheimer's
and Down's syndrome. Simply changing the shape of a region of the protein
results in a new and dangerous function.
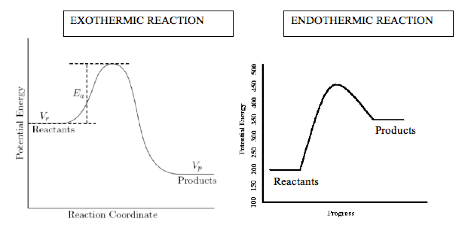
Reactions
change the covalent bonds within a molecule, breaking old bonds and making new
ones. If there is more energy released when the bonds form between the products
compared to the amount of energy absorbed to break the bonds between reactants,
then the reaction is termed exothermic, and heat is given off, and the products
are more stable than the reactants. The opposite is true for an endothermic
reaction, and heat needs to be added at the beginning for the reaction to
occur. This yields an unstable product at the end of the reaction. In class we
analyzed ATP and learned that ATP hydrolysis, ATP + H2O ¨ ADP
+ P, is an exothermic reaction, the water breaks the oxygen and phosphorus
bond, giving off essential energy for cellular functions.
References:
http://people.sps.lane.edu/jtyser/chem/Quiz/Unit12Test.html
(endothermic potential energy diagram)
http://www.simsoup.info/SimSoup/Potential_Energy_Profile.png
(exothermic potential energy diagram)
Cocchetto, A. (2004). Amyloids:
A Basic Primer. http://www.ncf-net.org/forum/amyloids.htm
http://en.wikipedia.org/wiki/Amyloid
(Amyloids)
http://en.wikipedia.org/wiki/Prion
(Prion)
Misfolding the key to proteinÕs ability to kill brain cells. Research
News. Ohio State University http://researchnews.osu.edu/archive/prpfind.htm