


Home MCEP home
E-portfolio CHEM502
ENERGY
OF ETHANE & BUTANE'S CONFORMERS
|
Introduction
Ethane and Butane Graphs
Ethane
and Butane
Excel Data
Discussion/ Comparison
Introduction:
Energy of
Ethane and
Butane Conformers
The conformers of ethane and butane have varying steric energies
depending upon the relative position of the bonds in the molecule and
the interactions that occur between them.
The purpose of this exercise was to model various conformers in Chem3D
(by changing the dihedral bond angle in 15 degree increments), and then
use the Molecular Mechanics (MM2) feature of Chem3D to calculate
various energies using classical mechanical approximations. The
minimum energy conformer for both ethane and butane, the anti-staggered
conformer with a dihedral bond angle of 180 degrees, was obtained by
manually adjusting the molecule and then using the
'Minimize Energy' feature. The following energies were recorded
and graphed using Excel:
1) TORSIONAL ENERGY--energy from the ROTATION of a bond away from its
optimal position (anti, staggered conformer)
2) 1,4-VAN DER WAAL'S ENERGY--energy from the STERIC REPULSION of
nonbonded atoms on adjacent carbons
3) non-1,4-VAN DER WAAL'S ENERGY--other STERIC REPULSIONS or
ATTRACTIVE INSTANTANEOUS DIPOLES within the molecule
4) TOTAL STERIC ENERGY--the sum energy associated with a particular
molecular conformer (from bending, stretching, torsion, and vanderwaals
interactions)
Graphs:
Click on a
chart for a closer
view.
Discussion/ comparison:
ETHANE
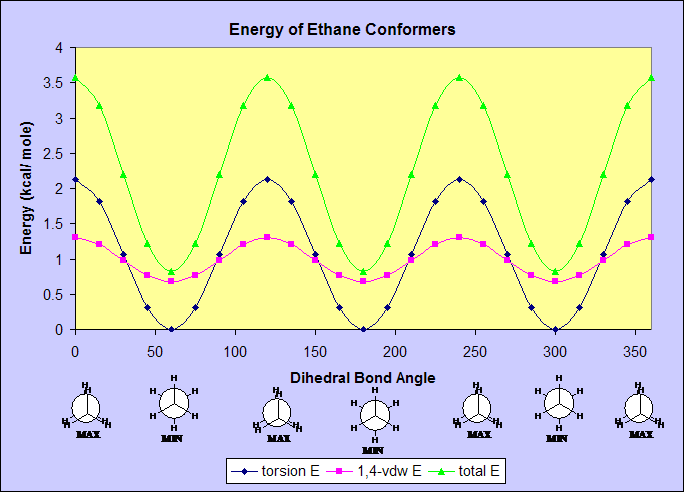
An analysis of the total energy of ethane's conformers shows a cyclic
cosine-like wave. The groups on the two carbons are all hydrogens
and therefore all equivalent. The highest energy conformer,
the eclipsed conformer, maximizes the repulsions
between the C-H bonds on one carbon and the C-H bonds of the adjacent
carbon, resulting in an energy of 3.5803 kcal/ mol. The staggered
conformer has a minimum steric energy of 0.818 kcal/ mol because it
minimizes the repulsions aforementioned. As the dihedral
angle increases, the molecule oscillates from the eclipsed (high
energy) conformer to the staggered (low energy) conformer every 60
degrees.
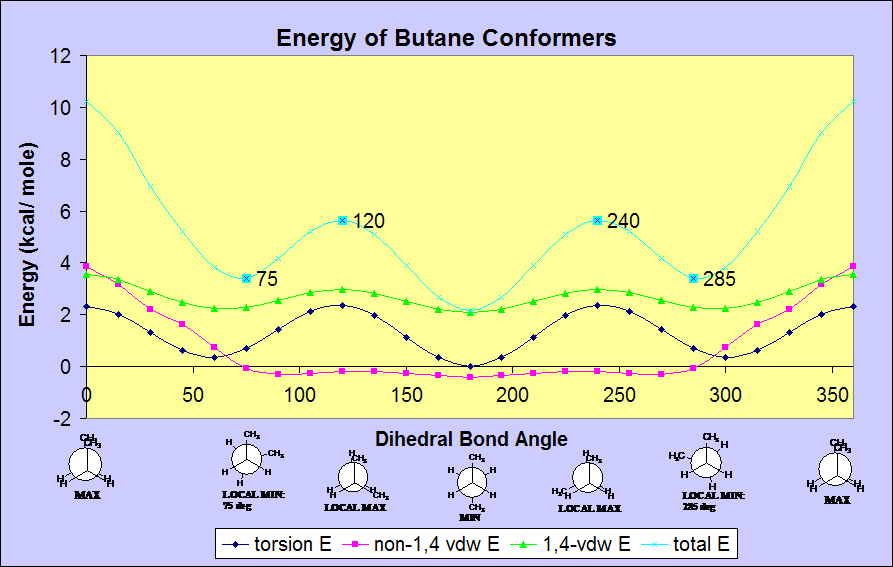
BUTANE
An analysis of the total energy of butane's conformers shows a more
complex wave because the groups on C2 and C3 are not all equivalent (as
in ethane); C2 and C3 have 2 H's and 1 CH3 (methyl) group attached to
them. There are, therefore, two types of eclipsed conformations
corresponding to the global maximum (10.212 kcal/ mol) and the local
maxima (5.6321 kcal/ mol). The
highest energy eclipsed conformation results when the bond and steric
repulsions are maximized by eclipsing the two larger methyl groups--at
0 and 360 degrees. The lower energy eclipsed conformation results
when the two methyl groups are eclipsed with hydrogens--at 120 and 240
degrees. The global minimum (2.1735 kcal/ mol), which occurs at
180 degrees,
corresponds to the anti-staggered conformer that places the methyl
groups as far as possible from each other to minimizes the repulsions
aforementioned. The local minima (3.4145 kcal/ mol) in butane
occur at 75 and 285 degrees.
COMPARISON
1) TORSIONAL ENERGY--The torsional energy for both ethane and butane is
about the same in
scale, however butane's torsional energy at 60 and 300 degrees
does not reach zero, as it does in ethane, because the methyl groups
still sterically interact
with each other, increasing energy.
2) 1,4-VAN DER WAAL'S ENERGY--The 1,4-van der Waal's energy
for ethane and butane is the same in pattern, but has a higher
magnitude in butane because of the larger relative size of C1 and C4 as
compared to H atoms. The larger size of the C atoms increases the
steric repulsion.
3) non-1,4-VAN DER WAAL'S ENERGY--The ethane molecule has 0 non-1,4-vdw
energy. Butane's
non-1,4-vdw energy between 75 and 285 degrees is slightly
negative, with local minima at 90 and 270 degrees, indicating a weak,
attractive, energy-lowering
interaction. The contribution of the non-1,4-vdw energy shifts
the local minima from the expected 60 and 300 degrees to 75 and 285
degrees; the C-H bond on C1 and the C-H bond on C4 repulse each other
more strongly at 60 and 300 degrees than at 75 and 285. It should
be noted that in this model of butane, only the C2-C3 bond is rotated
but the C1-C2 and C3-C4 bond are held stationary.
4) TOTAL STERIC ENERGY--See ethane and butane discussion above.




