Conformational
Analysis
of
cis-1,4-di-tertbutylhexane
Introduction: For
this assignment I was to use Chem3D to analyze the steric energies in cis-1,4-di-tertbutylhexane. While
I tried several different strategies to
get the proper structures, almost all of the strategies I used resulted
in
Chem3D converting my structure into trans
formations. My most successful strategy
was drawing the structure in ChemDraw, then pasting the structure into
Chem3D. This allowed me to calculate the
energies for the chair conformer and the twist-chair
conformer.
Once I had
recorded these, I
used the drag function on Chem3D to
move the individual carbon atoms to create a boat conformer. After I used
the clean-up function, I minimized
the energy, and was able to record the energies for the twist-boat
conformer. I was
never able to achieve a satisfactory minimized-energy conformer for the
boat conformer, as each time that I attempted
to minimize, Chem3D changed my structure into a twist-boat
conformer. The
energy I recorded for the boat
conformation is the one I recorded after I cleaned-up the structure,
but
without minimizing.
|
Chair Conformer
|
|
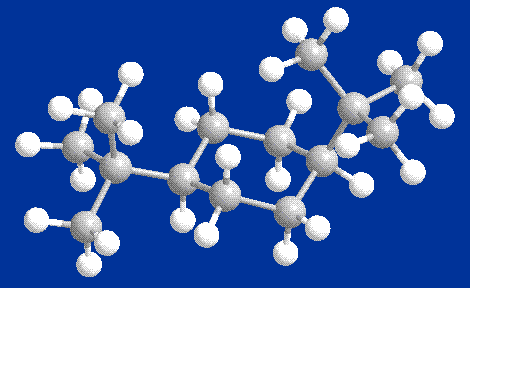
|
|
Stretch
|
2.505
|
|
Bend
|
6.0415
|
|
Stretch-bend
|
0.5685
|
|
Torsion
|
6.9886
|
|
Non-1,4
van der Waals
|
-1.8052
|
|
van der
Waals
|
10.3867
|
|
Total
|
24.6851
|
|
|
Initially, I expected the chair
conformer to exhibit the lowest steric energies. Cyclohexane,
with no substituted groups, exhibits highest stability (and lowest
energy) in the chair conformation. However,
when I created the structure with my molecular model kit, I began to
suspect that I was wrong. In the chair
conformation, while the 1-tertbutyl group can be equatorial (generally
the most stable, between axial and equatorial), the 4-tertbutyl group
is axial.
|
|
Twist-Chair
Conformer
|
|
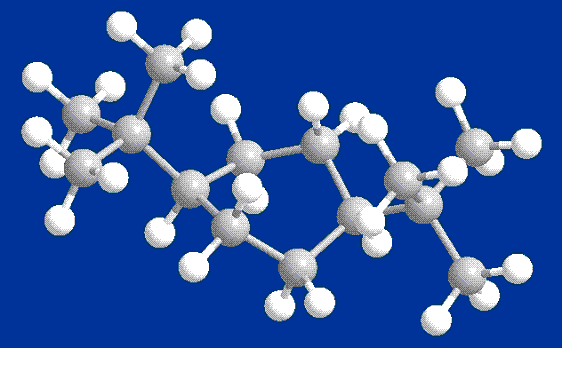
|
|
Stretch
|
2.4555
|
|
Bend
|
4.1294
|
|
Stretch-bend
|
0.5115
|
|
Torsion
|
9.4821
|
|
Non-1,4
van der Waals
|
-2.6172
|
|
van der
Waals
|
11.2032
|
|
Total
|
25.1646
|
|
|
I did anticipate that the twist-chair
conformer would have slightly higher steric energy than the regular
chair conformer. This is due to higher
torsion, due to the twisting of the structure. In
fact, we see that the stretch, bend, and stretch-bend energies are
slightly lower, because the twisting helps to alleviate the need for
the molecular bonds to stretch and bend. However,
as noted earlier, the torsion energy is higher. In
addition, the van der Waals energy is higher, resulting in a higher
total energy than the regular chair conformer.
|
|
Boat Conformer
|
|
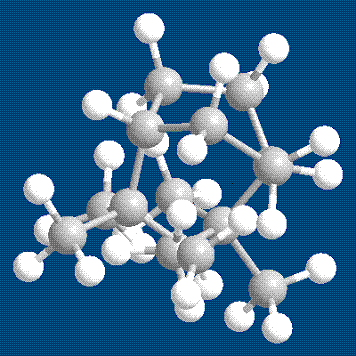
|
|
Stretch
|
0.0002
|
|
Bend
|
0.0059
|
|
Stretch-bend
|
0.0001
|
|
Torsion
|
18.1107
|
|
Non-1,4
van der Waals
|
4116.76
|
|
van der
Waals
|
23.5385
|
|
Total
|
4158.42
|
|
|
I also predicted that the boat conformer
would have the highest energy. If one just
looks at the a picture of the structure, it looks like a tangled mess. Without even doing calculations, it is clear
that torsion, van der Waals, and non-van der Waals are going to be
higher. When I completed the calculations,
the stretch, bend, and stretch-bend energies were significantly lower,
but as predicted, the torsion and van der Waals energies were
significantly higher. In addition, the
non-1,4 van der Waals skyrocketed, resulting in a total energy that was
exponentially higher than the other conformers.
|
|
Twist-Boat
Conformer
|
|
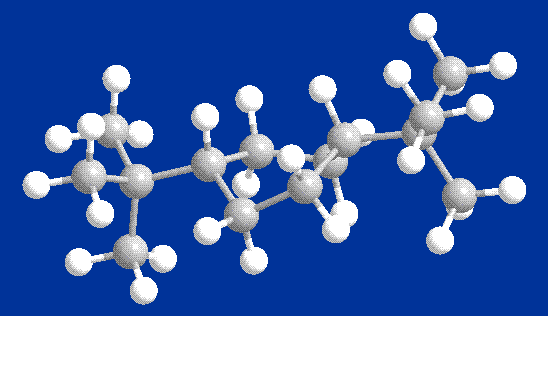
|
|
Stretch
|
2.3426
|
|
Bend
|
3.8716
|
|
Stretch-bend
|
0.4883
|
|
Torsion
|
8.4255
|
|
Non-1,4
van der Waals
|
-2.1531
|
|
van der
Waals
|
11.0035
|
|
Total
|
23.9784
|
|
|
Finally,
the twist-boat conformer turned out to be the
conformer with the lowest total energy. The
torsion and van der Waals energies were higher, due the twisting, but
everything else was lower, resulting in the lowest total energy.
|
Conclusion: In
the
end, as stated above, the twist-boat conformer was the conformer with
the
lowest total energy. This is
because,
in the cis conformation, the
twist-boat conformer is able to have both tert-butyl groups in an
equatorial
position, whereas in the chair conformation, only one tert-butyl group
was able
to be in an equatorial position. While
the 1-carbon and the 4-carbon are on the same side of the cyclohexane,
the
negative interaction of these is much less than having a large axial
group,
such as an axial tert-butyl. The benefit
of two equatorial tert-butyls outweighs the conflict of the carbons.
If this were an
analysis of trans-1,4-tertbutylcyclohexane, the
result would be much different. In that
case, both tert-butyl groups would be axial in the chair conformation.
K. Sundeen
Summer 2007