On the first day of Chem 505, the Environmental Chemistry class, we were given a pre-test. The questions reflected the material that we would cover during the class. I knew almost nothing about Environmental Chemistry, and this was reflected in my score (16/40), as can be seen below.

Question #30,
specifically, asked
about Eh. The question read, "Why is it important to
maintain a
high Eh in drinking water?" I had never heard of Eh before, so I
couldn't begin to explain why maintaining a high Eh in drinking water
is important. I put a question mark, and got a big "X" for my
effort, as can be seen below.


PIM #3 was a PIM we had to complete in Chem 505 (Environmental Chemistry). For this PIM we were required to research the events that led to high quantities of lead being found in the drinking water in Washington D.C. We were required to then write a brief paper about the chemistry behind the events. In particular, I would like to highlight the concluding sentences of my paper, seen below, which highlights my use of the Eh concept, in reference to the real-world of Philadelphia drinking water. The data to which it refers is the data from a lab we conducted that involved on-site sampling of the water in the Wissahickon, which is one of the sources of water for Philadelphia.

I learned the content in a series of lectures in Chem 505 (Environmental Chemistry). An image of the slide about Eh in the Washington D. C. water can be seen below.
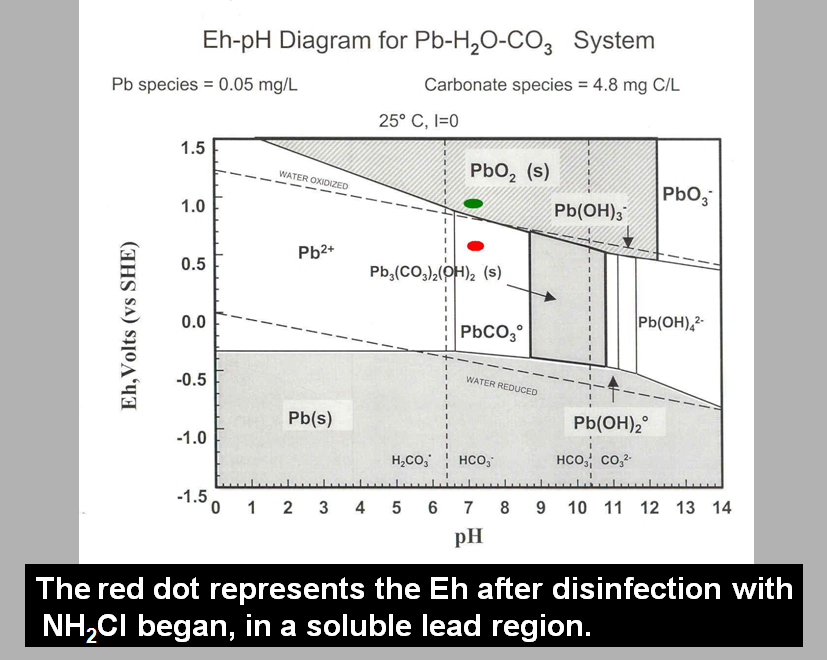
As I stated in the conclusion to
my PIM paper, the
Eh of the Wissahickon water sample was -.046109, and
the pH was 7.84. Given these numbers, it was clear to me that
there was a high liklihood that lead would be present as dissolved
lead, rather than as filterable precipitate or solids, thus my
statement that there would be "lead, lead, everywhere. . . ."
Previous to Chem 505, I did not even know what Eh was. After learning the content, I not only knew what Eh was, but could apply it to the situation found in the drinking water in Washington D.C., and ultimately make a statement about its application to the drinking water in Philadelphia.
Baseline for Artifact #2: Baseline for Mutter Research Paper (Chem 504 - Biochemistry)
In Chem 504, Professor Gelhaus had us visit the Mutter Museum, a museum located in the College of Physicians, and which houses collections of medical oddities, meant to educate doctors about different medical conditions, both common and rare. We were then assigned the task of picking something from the museum to research further. A baseline proposal was written to inform Professor Gelhaus of the direction of our inquiry. I chose to find out more about a proosed hair dye for women in the renaissance that used lead. Since I knew that lead is extremely dangerous (the exhibit was about the dangers of lead), I was curious as to what the benefits of such a dye could be. Here is an image of my complete proposal:
In this proposal, it is clear that I do not know what chemical processes would be most involved in the working of the hair dye. My research centered around finding out more about these chemical processes.
These are two images of excerpts from the resulting paper. The first is an image of the section that discusses the role of lead in producing the active ingredient, lead acetate.

The second is an image of the section that discusses the role of fatty acids in precipitating lead acetate crystals out of solution, as well as the resources I used in writing the paper.
I had actually learned about the lead acetate reaction in both Organic, and Inorganic Chemistry. In doing the research, the previously-learned information was refreshed. Furthermore, the research I conducted introduced me to new information about the use of fatty acids in precipitating the lead acetate, as well as the result of the reaction of lead acetate with the sulfur in the hair (a component of which we learned about in Biochemistry).
Before completing the course work in Organic Chemistry and Inorganic Chemistry, as well as the research for this paper, I would not have thought twice about what makes Just For Men, and similar dyes, work. Obviously, from my baseline paper, I would never have guessed that it had anything to do with lead. Now, whenever I see the commercial, I think to myself, "Lead??? Really????"
Baseline for Artifact #3:
In the website that I put together for Chem 507 (Spectroscopy), we were asked to have a page that discussed practical applications of the topic that we chose to research. I chose to examine bird and insect eyes as spectrometers. My practical application page was about enhancing the range of visible wavelengths for humans. In it, I make reference to a common misconception that people have, fed by movies and television shows, that thermal imaging "requires the subject being observed to be hot, and that one could avoid detection simply by lowering the temperature of the object." This is a misconception that I held, again, fed by movies and television shows.
This webpage discusses actual technologies that we use to improve the range of visible wavelengths. To access this particular page on the website, click here.
In the webpage, I discuss several things that I learned in my research about visible wavelengths, and the practical applications of an understanding of wavelength, and how the wavelength absorbance and transmittance are interpreted by eyes. First, it is necessary to understand why the eye can be seen as a spectrometer.
In Chem 507, as well as in Inorganic Chemistry (held the previous summer), we learned about the role of absorbance and transmittance at various wavelengths in the perception of color. Below is an image of an excel spreadsheet from a lab we completed in Inorganic Chemistry that measured absorbance both with a Spec 20, and visually (we actually completed a similar lab to this in Spectroscopy class).
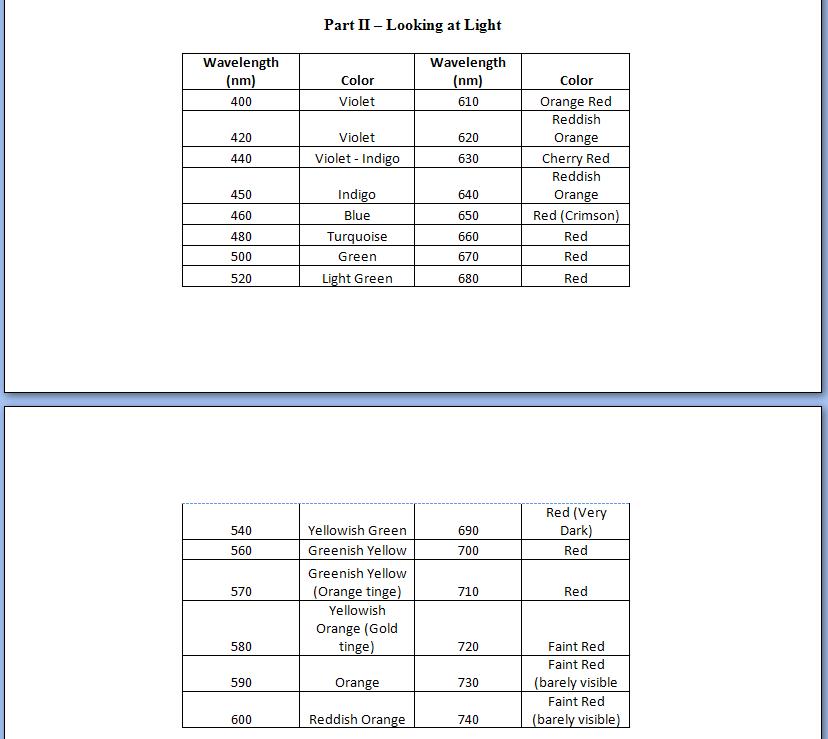
This next image is an image of a
table from a lab we completed in Spectroscopy class that compared color
with wavelength measurements in a Spec20.
These are only two examples of the
many ways that color and wavelength were discussed in both Inorganic
Chemistry and Spectroscopy. All together, it
helped me to understand the relationship between wavelength and color,
and when it came time to pick the topic for my Spectroscopy website, I
decided I was able to determine that eyes are a lot like
spectrometers. They collect wavelength data, and translate
them into information.
Since I learned in my research that birds and insects have a much wider range of visible wavelengths, I was interested in finding out how humans could, or do expand their visible range. I learned that the current technology centers primarily in expanding visibility into the infrared range (longer wavelengths, lower energies than normal human range). I also learned that, in addition to thermal imaging (which detects, collects, and translates variations in wavelengths into images), a type of goggle called Night Vision Devices use image enhancement technology, which collects photons, and translates the photons into recognizable images. Again, to view this portion of the website, go here.
Since I learned in my research that birds and insects have a much wider range of visible wavelengths, I was interested in finding out how humans could, or do expand their visible range. I learned that the current technology centers primarily in expanding visibility into the infrared range (longer wavelengths, lower energies than normal human range). I also learned that, in addition to thermal imaging (which detects, collects, and translates variations in wavelengths into images), a type of goggle called Night Vision Devices use image enhancement technology, which collects photons, and translates the photons into recognizable images. Again, to view this portion of the website, go here.
I went from holding the common misconception that many people have about thermal imaging, to truly understanding how current technology can expand the range of visible wavelengths for humans. In addition, the project solidified my understanding of the relationship between energy, wavelengths, and our visual perceptions.