X-Ray Spectroscopy and The Development of the Periodic Table
 |
 |
 |
 |
 |
 |
 |
 |
History
Periodic
Table:
The
development of the periodic table generated numerous ideas emanating
from
seemingly endless directions but mainly promulgated by the contribution
of five
scientists. These men made independent contributions which lead to the
first
periodic system created by Mendeleev in 1869. In 1862 De Chancoutois
presented
a system which determined that element’s properties are a function of
their
atomic weights. Scientists like Odling in 1864 arranged the elements by
increasing
atomic weight. Meyer then became the first scientist to actually
publish a
periodic table in a textbook. The table Meyer developed was different
from the
previous tables because he used physical properties to help develop his
periodic system and did not place as much emphasize on atomic weight as
in
previous systems. In 1865, Newlands developed the Law of Octaves which
showed
that after every seventh element the properties start to repeat. Then in 1866, Hinrichs showed a relationship
between the elements based on the size of their atoms.
All of these contributions and his own
research lead to the development of the first periodic system in 1869
by
Dimitri Mendeleev.
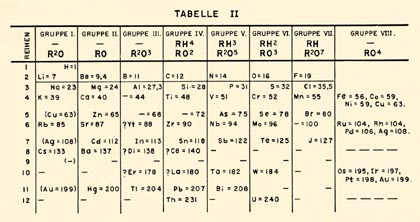
Mendeleev’s Original Table from 1871
http://facweb.eths.k12.il.us/chemphys/Word/Chem-Phys%20Quantum%20Numbers%20-%20Student_files/image001.jpg
Mendeleev’s
table was divided into both main and sub-groups. Mendeleev also left
blank
spaces for elements that had not been discovered and also predicted
some of the
properties of those undiscovered elements. There are several different
theories
as to why Mendeleev’s periodic system became the standard in the
scientific
community but the focus of this project is not Mendeleev’s
table but how x-ray spectroscopy
help lead from Mendeleev’s table to the table developed by Henry
Moseley in
1913.
Discover
of
X-Rays and X-Ray Spectroscopy:
In 1895, a
German physics professor Wilhelm Conrad Rontgen made one of the
greatest
breakthroughs in the world of science. Rontgen was working in his lab
with a
Crookes
tube (a tube that has a cathode at one end and an anode at the
other
end with no air in the container) studying the emission of ultraviolet
light
using barium platinocyanide crystals. When Rontgen placed a voltage
through the
tube it would produce a fluorescent glow. During his experiments,
Rontgen
noticed that from a distance his tube produced a green glow when
wrapped in
black paper. The green glow was produced on a screen a few feet away
from the
tube. Rontgen determined this glow was produced from glass at the end
of tube
where the cathode beam had struck. This
showed him that the material he was using was able to produce a glow
through
the dark paper that covered the tube.
Rontgen at first was shocked by his discovery and continued to
repeat
the process over and over again but moving the screen further and
further away.
Every time he ran the experiment with the screen at a greater distance,
he
continued to produce the same result. Rontgen had realized that he had
made a
major breakthrough. Rontgen knew from
previous work that the cathode rays could not produce fluorescent glow
and
neither could the visible light because the tube was covered with dark
paper.
From this knowledge he realized some type of radiation was producing
the
fluorescent light and he was now determined to delineate the cause.
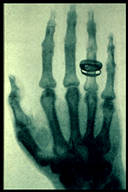
Rontgen spent about eight weeks continually working on his new discovery. Over this period he continued to place different objects between the tube and the screen. Each time another image would be produced. Depending on the thickness of the object the intensity of the fluorescent effect would vary on the screen. After weeks of experimentation, Rontgen decided to substitute a photographic plate for the screen and had his wife Bertha place her hand on the plate. He began to generate beams aimed at his wife’s hand. After about 15 minutes the image of her bones and her wedding band was produced on the screen. This is the first record of a human x-ray. The image below shows the x-ray of Rontgen’s wife’s hand:
Rontgen had now decided to
publish
his work; not exactly sure what was producing these rays. He coined the
term
x-ray because in math X is used to describe an unknown term. His
initial work
and publication is what lead to the development of x-rays and the
future
development of the atomic structure. In 1901, Rontgen won the very
first Noble
Prize for his work. If not for Rontgen’s
discovery of x-rays J.J. Thomson and
Ernest Rutherford may not have made the discoveries of the electron,
proton,
and nucleus. These discoveries were the fruits of Rontgen’s seeds.
Charles
Barkla and Ernest Rutherford:
Physicist
Charles Glover Barkla and Physicist Ernest Rutherford worked on the
theory of
atomic charge. They each took a different path in their experiments but
it
seems their conclusions are almost identical. Barkla started to analyze
x-ray
scattering from different elements. From previous work, it had been
determined
that the x-rays emitted from the tube were heterogeneous and depended
on the
material within the tube. Barkla took this knowledge and through
research
determined how to produce homogeneous radiation of elements from the
tube. This now showed that elements had
their own
characteristics in the line spectra of x-rays (for more information
click "Spectroscopy Page Link" .
It had also showed that a second emission was determined to be
one of
two types. The first kind of emission was when the x-rays were
scattered they
were unchanged and the second kind was the fluorescent radiation
produced was
specific to the substance being used. After Barkla made this
determination from
homogeneous radiation he classified the two series as K-Fluorescent and
L-Fluorescent radiations. This discovery led Barkla to determine that
each
element had its own x-ray characteristics in the x-ray spectrum. His
work in
determining that elements had their own x-ray characteristics was the
first
major breakthrough in the development of x-ray spectroscopy and he was
awarded
the Noble Prize in 1917 for his work.
Ernest
Rutherford was working with alpha particles and the atom. Rutherford
had two
students performing experiments with alpha particles. Hans Geiger and
Ernest
Marsden both student’s of Rutherford would fire a stream of positively
charged
alpha particles at a piece of thin foil composed of gold, they started
to
realize that the particles would scatter, and some particles would come
right
back in the direction which they were launched. From Thomson’s previous
work
with the atom, it was presumed that the alpha particles should pass
through the
foil. After repeated attempts, Rutherford now drew the conclusion from
his
student’s work, that in the center of the atom there was a small dense
concentrated region which held positive charges, and the negative
charges would
make up the rest of the volume of the atom. Rutherford also determined
from
these experiments that the charge on the atom is half of the atom’s
atomic
weight. Rutherford and his associates also start performing experiments
on
elements that ranged from aluminum to lead and from all these results
he
concluded that the degree of scattering is proportional to the square
root of
the atomic weight of the specific atom. After making these two
conclusions he
was now drawn to the final understanding that the scattering was
actually
proportional to the square of the atomic charge because alpha particles
would
actually being scattered by the nuclear charge and not the atomic
weight.
William
Henry Bragg and William Lawrence Bragg:
William
Henry Bragg and his son William Lawrence Bragg also helped in the
development
of x-ray spectroscopy. There research was another major breakthrough in
the
development of x-ray spectroscopy. Bragg’s work was in the development
of x-ray
diffraction of crystal surfaces at different angles. Since
the structure of a crystal can be determined by examining the
diffraction
pattern of a beam of radiation incident on the crystal.
When examining the patterns , the focus is
the directions of the diffraction and the corresponding intensities. These pieces of information are of paramount
importance to determining the crystal structure responsible for the
diffraction.
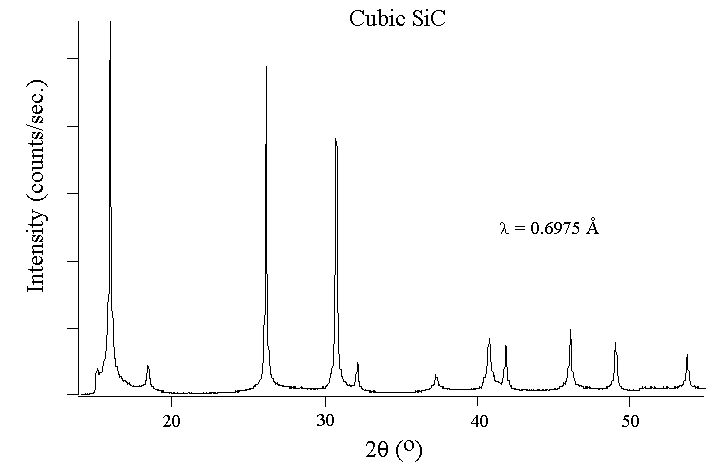
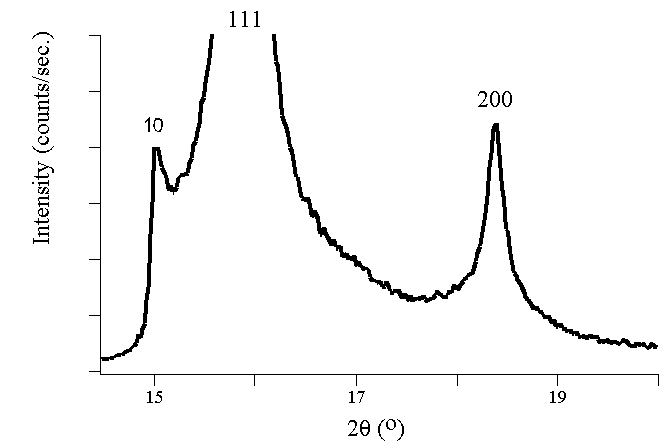
For
the two diagrams above, the peaks represent Bragg's peaks which are
indicative of the reflection spots observed in the diffraction image.
The amplitude of the peak corrsponds to the intensity of the
diffraction. This is an example of constructive
interference because the crystal converges weak scatterings into a
much more disctive coherent reflection that is shown here.
Both father
and son won the Noble Prize in 1915 for their development of
determining
crystal patterns. Even more importantly their research combined with
the
research of Rontgen, Barkla, Rutherford, and Bohr led Henry Moseley to
the
final development of the Atomic number and conformation of the periodic
table.
Below is an imagine of the
original Bragg Spectrometry:
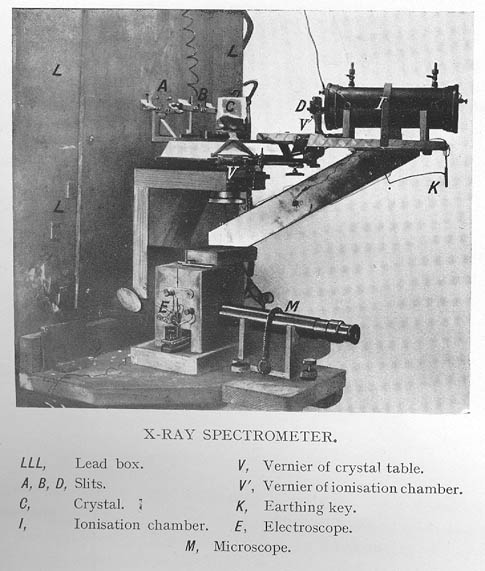
http://www.leeds.ac.uk/library/spcoll/bragg-notebook/images/fig2a_large.jpg