
X-Ray Spectroscopy and The Development of the Periodic Table
 |
 |
 |
 |
 |
 |
 |
 |
Moseley

http://www4.nau.edu/microanalysis/microprobe/img/Moseley.jpg
Henry Moseley is credited with one
of the most important discoveries in the field of chemistry. At a very
young age of 26, Moseley showed how each element's atomic number was
greater by one unit in the periodic table. Using the skills
Moseley developed, he was able to confirm Mendeleev's periodic table
and other issues that continued to rise with the periodic table.
Moseley helped fill in the gaps that remained and also predicted there
were seven gaps that had been undiscovered. These gaps were atomic
numbers 43, 61, 72, 75, 86, 87, and 91. Moseley's method brought a new
light to the periodic table and helped answer a lot of issues that were
plageing scientist before him such as the location of tellurium and
iodine, and the location of cobolt and nickel. Moseley's life was cut
short when he died during World War I. Excluding a small group of
scientist, Moseley was not very well known in his field but his work
that he left behind made him a legend in the field of Physics forever.
Moseley started his career off working under Ernest Rutherford. Rutherford could tell early on that Moseley had great potential in the world of physics. After working with Rutherford, Moseley moved on to try and test van den Broek's work containing the organization of elements by their atomic number. Moseley had come into close contact with a young Niels Bohr and was well aware of Bohr's work with atomic spectra. Moseley developed an apparatus to test the K alpha X-rays would be measured. When Moseley first started to test his experiment, he used 14 different elements. Out of the fourteen elements he chose, nine of the those elements were determined to have a contineous series on the periodic table. Those nine elements are from titanium to zinc.
The diagram below was the chart Moseley created by plotting the frequency of the lines from the K series of the spectral lines that were produced by his experiment. He then determined from this plot that the frequencies of lines from the K series of spectral lines was directly proportional to the square of the integer. When this discovery was made Moseley started to quickly realize his results were showing how each element's K series of Spectra Lines was showing the position of each element on the periodic table in increasing order of one unit.
Moseley started his career off working under Ernest Rutherford. Rutherford could tell early on that Moseley had great potential in the world of physics. After working with Rutherford, Moseley moved on to try and test van den Broek's work containing the organization of elements by their atomic number. Moseley had come into close contact with a young Niels Bohr and was well aware of Bohr's work with atomic spectra. Moseley developed an apparatus to test the K alpha X-rays would be measured. When Moseley first started to test his experiment, he used 14 different elements. Out of the fourteen elements he chose, nine of the those elements were determined to have a contineous series on the periodic table. Those nine elements are from titanium to zinc.
The diagram below was the chart Moseley created by plotting the frequency of the lines from the K series of the spectral lines that were produced by his experiment. He then determined from this plot that the frequencies of lines from the K series of spectral lines was directly proportional to the square of the integer. When this discovery was made Moseley started to quickly realize his results were showing how each element's K series of Spectra Lines was showing the position of each element on the periodic table in increasing order of one unit.
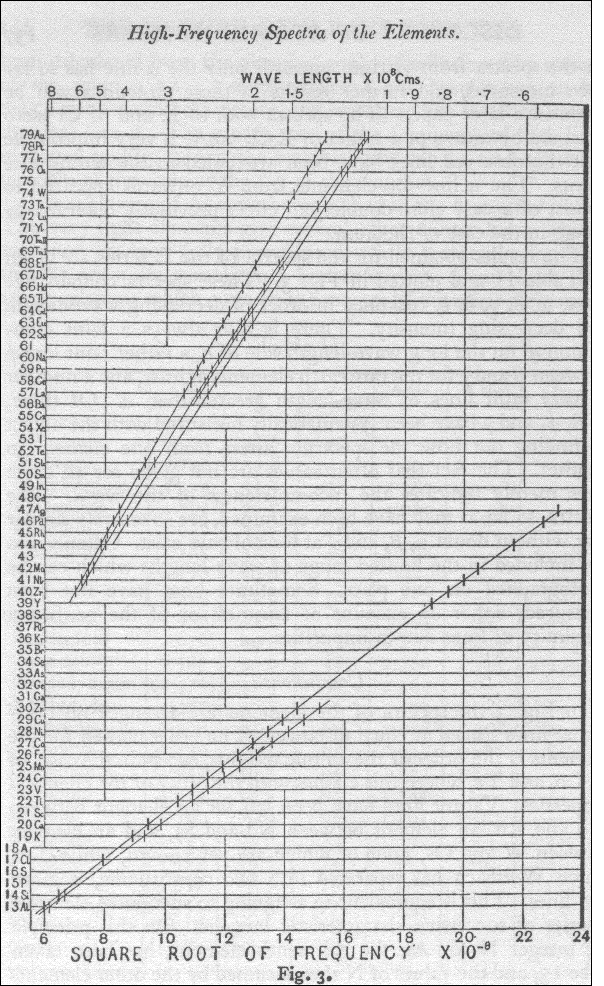
http://lhs2.lps.org/staff/sputnam/chem_notes/Unit3%20Periodicity/Moseley-Fig3.gif
In 1913 Moseley published his one of two famous papers describing the work he had completed compairing the K series of spectra lines with the square root of their frequencies. He explained in this paper how his work lead him to the conclusion that with his new equation now known as Moseley's Law (in the image below) that he could safely predict the order of elements in the periodic table by their atomic spectra.
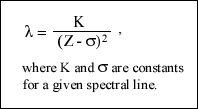
In 1914, Moseley published his second famous paper which also turned out to be his last paper. Moseley now set his sights on examing 30 more elements to determine their atomic spectra and location on the periodic table. He looked at the K series from aluminium to silver and the L series from zinc to gold. He looked each element's spectral lines and finally made the major conclusion he had been working on with this process. He had finally and conclusively determined the importance of atomic number and how each element should be arranged in the periodic table by their increasing atomic number.
Once Moseley had made this breakthrough, he wanted to continue his work by help filling in the gaps of Mendeleev's original table. Many chemist claimed they had the element that was one of the 16 missing elements from Mendeleev's table. Moseley using his technique examed close to 70 so called elements that should be on the periodic table. Moseley used his work with spectral lines to show that majority of the elements did not fit the qualifications and were not the elements that belonged on the periodic table. In fact he showed they were not pure elements by themselves because they did not have atomic number properties.
A famous chemist named Georges Urbain had become aware of Moseley's work and wanted to test the theory himself. He went to meet Moseley to have him show him how accurate his theory truely was. Urbain gave Moseley a sample which contained a mixture of elements. Within a short amount of time, Moseley was quickly to identify the elements in the mixture by their spectral lines. Moseley accomplished this in a few hours and Urbain accomplished the same results but it took him nearly six months of chemical analysis.
Moseley's work had clearly redefined the world of both Physics and Chemistry. There had seemed to be a clear cut method on determining not only the order of elements in the periodic table but their atomic numbers but also there was now a method to identify elements in a chemical mixture. Moseley's work led to a change in the world of chemistry and opened the door for so many more breakthroughs and discoveries to occur.
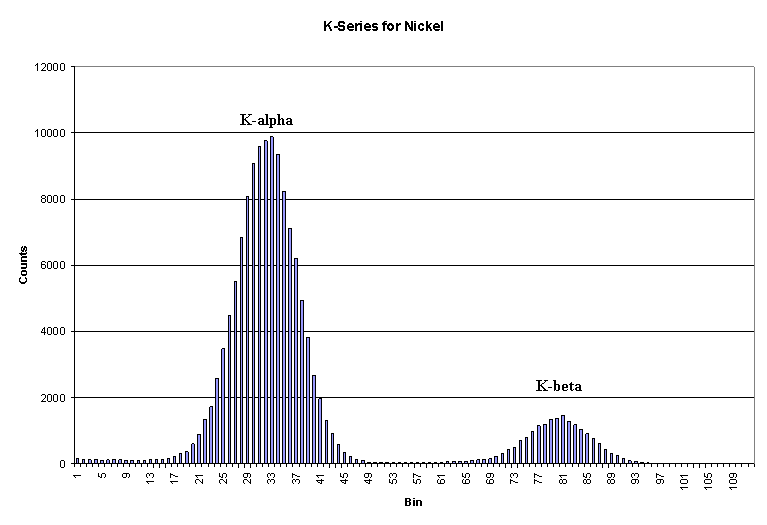
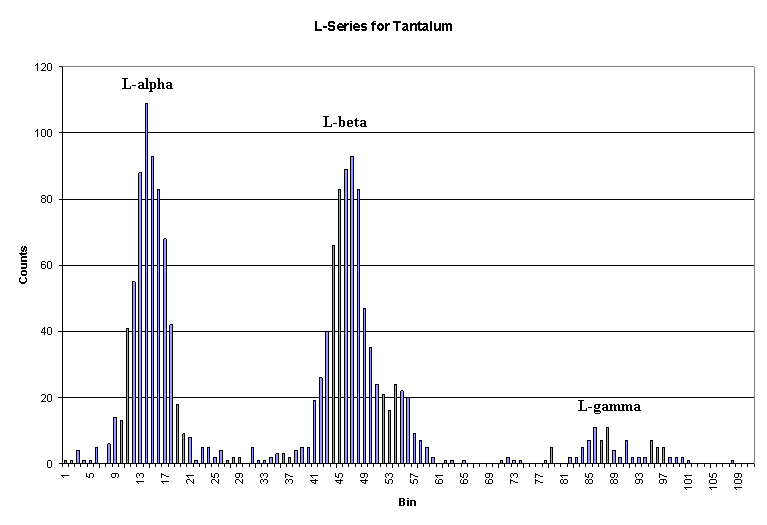
The two diagrams above represent an example of both K Series Spectral Analysis and L Series Spectral Analysis for two different elements. These are how the analysis is performed today but this was the same process Moseley used in the early 1900's to determine the correct order of the periodic table. Moseley's original spectral analysis chart is shown above.