
X-Ray Spectroscopy and The Development of the Periodic Table
 |
 |
 |
 |
 |
 |
 |
 |
Spectroscopy Information
What is X-ray Spectroscopy?
Immediately
upon the discovery of x-rays in 1895, Rontgen also discovered the most
important
use for x-rays. With his hand in front
of the machine he saw his carpals and metacarpals displayed on the
screen. While fascinated by this striking
discovery,
one can’t help but ask how it works.
Most types
of spectroscopy rely on vibrational, rotational and translational
movement of
bonds in a molecule or atoms. However
x-ray spectroscopy is different in that electron transitions are
involved. Usually energy is absorbed by an
electron and
it moves to the excited state and upon return to the ground state, a
photon of
certain energy and wavelength is emitted as shown below.
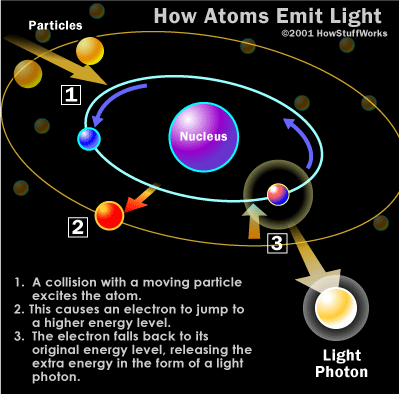
http://health.howstuffworks.com/x-ray1.htm
This concept
still holds true x-rays are unique in that the electrons which are
excited are
not valence electrons. The collisions
with energy particles are so powerful that the inner shell core
electrons are
excited. This causes an inner orbital to
be vacant therefore an outer electron drops to a lower orbital. This drop in energy of an electron causes the
x-ray photons to be released. The figure
below illustrates this.
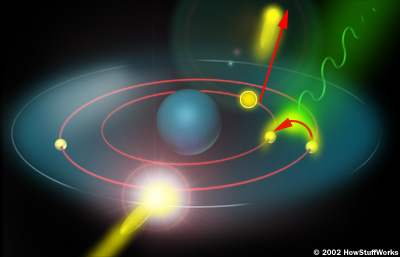
http://static.howstuffworks.com/gif/x-ray-atom2.jpg
Figure 2 (above) shows a free electron colliding with a tungsten atom knocking one of the inner shell electrons out of a lower orbital. A higher orbital electron fills the empty position releasing excess energy.
This is an
example of primary excitation. The high
energy particles that excite the inner shell electrons are electrons in
this
case. Secondary excitation or Fluorescence excitation is caused when a
photon
hits an inner shell electron allowing it to reach the excited state and
another
electron drops down to fill the opening releasing less energy than was
absorbed. This is the premise of
fluorescence
and why x-ray emission can be seen. As
an example, a 1s electron is excited because it is bombarded with a
photoelectron during primary excitation.
This electron is excited from the K level below which is the
notation
for 1s.
In
1913 Bragg and Bragg used Barklas and Sadler’s data to do many x-ray
experiments using different crystals as a three-dimensional diffraction
grating. They came up with a law that
allows one to calculate details
about the
crystal structure, moreover, if the crystal structure is known, to
determine
the wavelength of the x-rays incident upon the crystal.
For the diagram below:
Bragg’s
Law identifies the angles of the incident radiation relative to the
lattice
planes from which diffraction peaks occur.
Bragg derived the condition for constructive interference of the
x-rays scattered
from a set of parallel lattice planes (represented by the blue dots
shown in
the diagram below).
Bragg
considered crystals to be composed of parallel planes of atoms. Incident waves reflect only a small fraction
of radiation. The diffracted beams occur when the reflections from
different planes of atoms interfer constructive.
The equation below
quantatively represents Bragg's Law. For the equation n represents the
number of wavelengths, d represents the distance between the planes of
atoms and Θ represents the incident angle of reflection.
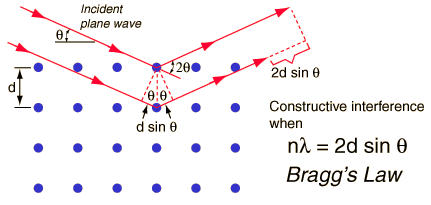
http://hyperphysics.phy-astr.gsu.edu/hbase/quantum/bragg.html
Moseley used this information
to show that wavelengths were not
only a characteristic of the element the target was made of, but also
they had
the same sequence as the atomic numbers. This allowed atomic numbers to
be
determined with certainty for the first time.
Soon after it was also
established that secondary fluorescent
x-rays were excited in any material irradiated with beams of primary
x-rays. This
started investigation into the possibilities of fluorescent x-ray
spectroscopy
as a means of qualitative and quantitative elemental analysis
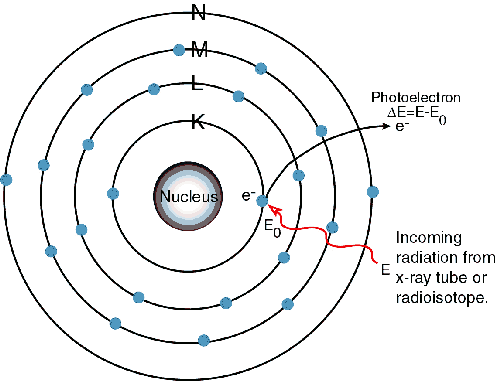
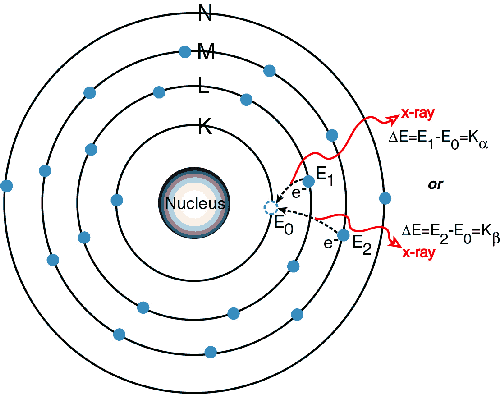
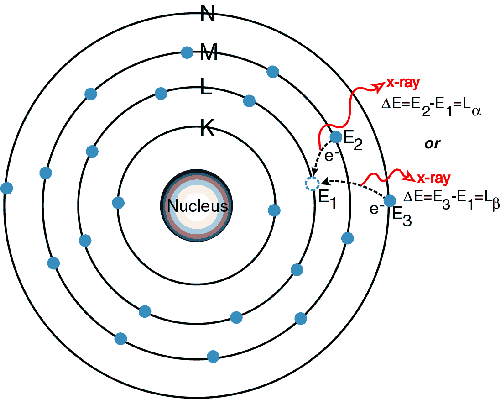
Since there
is now a vacancy on the K level shown in the 2nd picture, an electron
from the L or M level will replace it creating a new vacancy and yield
a photon
in the x-ray range. Since it is lower in energy than the initial x-ray
absorbed
it will fluoresce. New vacancies will be
created on the L or M level and new electrons will occupy the vacancies
releasing lower energy creating the L lines and this pattern will
continue. The criterion that must be met
in order for this to occur is that an absorption limit must be met. This means that the initial x-ray must have
the minimum energy required to excite an electron from a given
one-electron
state, which also corresponds to a minimum wavelength requirement.
Each atom
because it has a different electronic structure will emit a
characteristic
x-ray line emission spectrum. This is
one way in which x-ray spectroscopy can be used to quantitatively
measure
elements in a sample. The two types of spectrometers that accomplish
this are
wavelength dispersive and energy dispersive.
The
former system uses a diffraction crystal to focus specific
wavelengths onto a detector, the latter focuses all emitting x-rays
onto an
energy analyzing detector. Wavelength
dispersive spectrometers are the preferred instrument due to their
heightened
sensitivity. The diffraction crystal,
standard being lithium fluoride crystal, is rotated and the x-ray
emission is
focused on the detector.