
X-Ray Spectroscopy and The Development of the Periodic Table
 |
 |
 |
 |
 |
 |
 |
 |
Theory of Instrumentation
Development of
Spectrometers
X-rays
are used to
separate chemical components of a sample into their characteristic
spectral
lines for identification and determination of concentration.
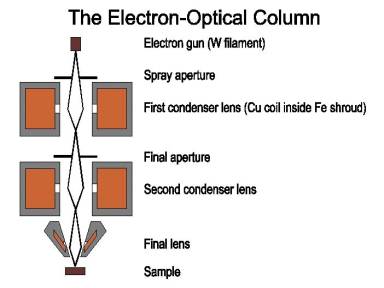
http://fp.okstate.edu/catlos/eprobe/working.htm
High
velocity electrons
are generated from a filament, in this case, tungsten.
These electrons are focused through condenser
lenses into a narrow beam. When the
electron beam strikes the same a characteristic spectral is obtained. This is used to obtain chemical
compositions.
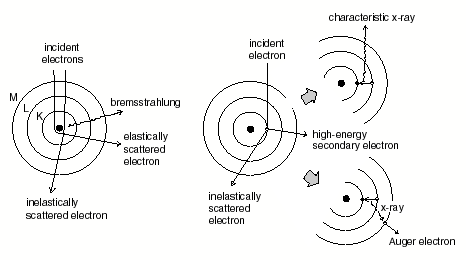
http://fp.okstate.edu/catlos/eprobe/working.htm
This
figure above shows how
the incident beam of electrons affects the sample and causes it to emit
x-rays
which dependent on the composition of the sample will by unique. The amount of x-rays emitted is a direct
reflection of the concentrations of elements in a sample.
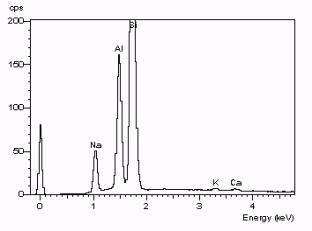
http://fp.okstate.edu/catlos/eprobe/working.htm
The
image above is an example of an
x-ray spectra of albite. Each peak
on the
spectrum represents a transition with a characteristic energy. Every
element in albite has its own
"fingerprint" of peaks. One
can see the characteristic peaks for
different elements and the amplitude of the peaks indicates the
concentration
of the respective element. For example
one can deduce from this spectra that not only is Silicon present in
this
sample but it is the most abundant element as well.
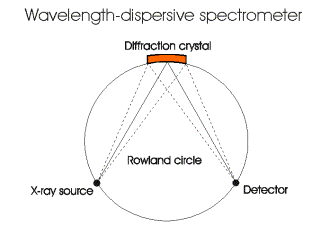
A
wavelength dispersive spectrometer collects data when the source
electron beam
hits a diffraction crystal, which disperses X-rays by Bragg reflection.
Most
electron microprobes are equipped with several different crystals to
allow
analysis of a wide range of X-ray wavelengths lithium fluoride,
pentaerythritol
and thallium acid phthalate are common crystals used. These crystals
can
measure all X-ray wavelengths generated by elements from 11Na
to 92U.
For lighter elements, wider d-spacing is needed. In this case, soap
films can
be used.
Current Instruments:
Below are examples of instruments used in x-ray spectroscopy

www.amptek.com/x123.html
This is an example of data of Multi-Element Fluorescence from Cd-109
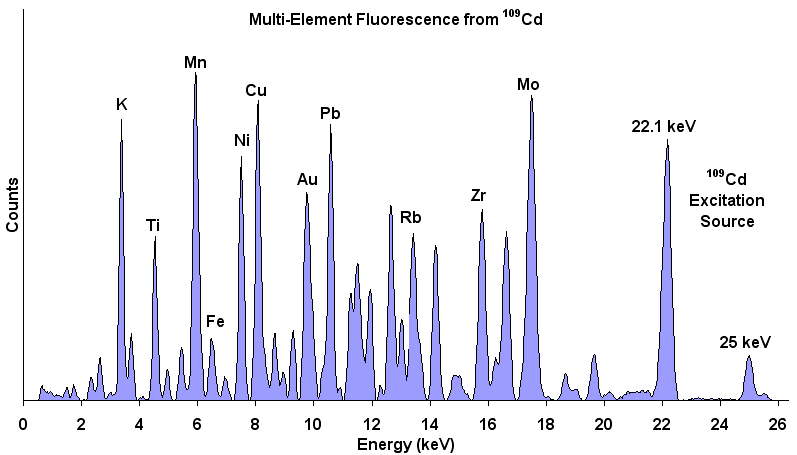
www.amptek.com/x123.html