Fluorescence ,
phosphorescence
(photoluminescence) and chemiluminescence
First off, let's talk about
luminescence
in general. Luminescence is the emission of light by a substance.
It occurs when an electron returns to the electronic ground state from
an excited state and loses it's excess energy as a photon.
Bioluminescence
is when the reaction happens in a living organism. In order to
understand
this better, we need to understand what is meant by a singlet and
triplet
state.
The electronic states of most organic
molecules
can be divided into singlet states and triplet states.1
Singlet state: All electrons in the
molecule
are spin paired. It is called a singlet because there is only one
possible
orientation in space.
Triplet state: One
set of electron spins is unpaired. It is called a triplet because there
are three possible orientations in space with respect to the axis
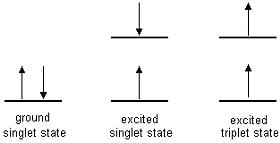
When we look at
excited
singlet states, one of the paired electrons from the ground state moves
to an excited state but does not change spin. (So
what is spin?) When something happens to the molecule like a
collision
with another molecule, the electron in the excited state could have a
spin
inversion. Now, we see an excited triplet state. The problem with this
spin
flipping, now the electron cannot return to the ground state until
its spin is flipped again. Otherwise, Pauli exclusion principle that
all
electrons must have a different set of quantum numbers would be
violated.
Now that we understand this aspect, lets look at how this relates to
fluorescence
and phosphorescence.
Fluorescence
Absorption of UV radiation by a molecule
excites it from a vibrational level in the electronic ground state to
one
of the many vibrational levels in the electronic excited state. It will
now be in an excited singlet state. (see above) This can be show
below by the blue arrow #1. The molecule can then undergo vibrational
relaxation
which is caused by a radiationless transition. This can occur several
ways2,3.
(1) emission of an infrared photon to go to a lower excited vibrational
state (2) transference of vibrational energy to another molecule by
collision,
to a different vibrational mode within the same molecule or to
rotational
motion in the same molecule. Once the molecule has reached the lowest
vibrational
state in the excited state, the molecule will release a photon (of less
energy than absorbed) to return to the ground state giving a wavelength
in the visible spectrum. What is seen is fluorescence (shown by blue
arrow
#2)

( from
http://www.shu.ac.uk/schools/sci/chem/tutorials/molspec/lumin1.htm)
Phosphorescence
When the molecule is in the excited
singlet
state, another possibility can occur. Sometimes, through collisions,
the
spin quantum number can be changed producing an excited triplet state.
(why?
)When
this happens, the term
intersystem crossing is used. The triplet
state usually is of lower electronic energy but higher vibrational
energy
than the singlet state it came from. This is due to the lower
interelectronic
repulsion in the triplet state 4,5 For this to happen, the
molecule
should have the vibrational levels of these two states (excited singlet
and excited triplet) overlap. This is why only some molecules show
phosphorescence.
The molecule will become trapped in this state, since returning to the
ground state will give two electrons of the same spin. The molecule
could
still lose vibrational energy to bring it down to the lowest excited
vibrational
state.
Chemiluminescence
When a chemical reaction results in an
electronically excited species like the deoxetanone in the firefly
reaction,
(see
mechanism)
the emission of a photon is called chemiluminescence. Once the excited
state is achieved, phosphorescence or fluorescence can occur. Because
the
reaction is occurring in a living organism, it is labeled
bioluminescence.
References
1. Image
fromhttp://www.shu.ac.uk/schools/sci/chem/tutorials/molspec/lumin1.htm
2. Moog,R.S., Spencer, J.N. and Farrell, J.J. (2004) Physical
Chemistry: A Guided Inquiry Atoms,Molecules and Spectroscopy,p 213.
3. Sharma, A. and Schulman, S.G.(1999). Introduction to
Fluorescence Spectroscopy. John Wiley and Sons: New York, p18-19.
4. Levine, I.R. (2002). Physical Chemistry, 5th ed. McGraw
Hill: New York, 801
5. McHale, J.I. (1999). Molecular Spectroscopy, first ed,
Prentice Hall: NJ.


