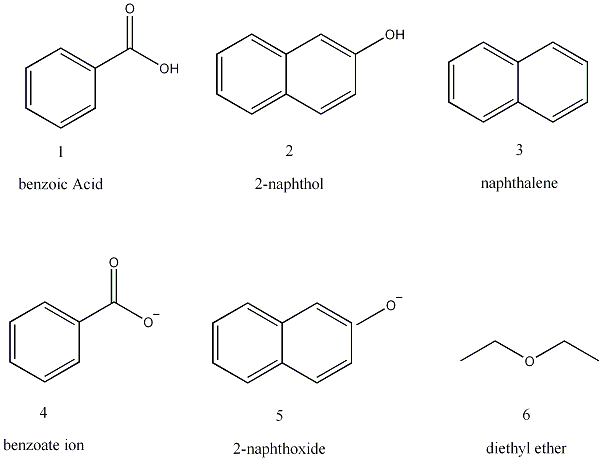
The purpose of this experiment was to perform a two-base extraction, thereby separating an “unknown” mixture of benzoic acid, 2-naphthol, and naphthalene into its separate components and determining the percent composition of the mixture. This separation was performed using a liquid-liquid extraction. The compounds were separated on the basis of the solubility of the acids and that of their salts. Stronger acids, in this case the carboxylic acid benzoic acid, can be converted to their salts through a reaction with a relatively weak base in comparison to phenols. The weak base that was used in this case is sodium bicarbonate. A stronger base, for example sodium hydroxide, is needed to convert the weaker phenol (2-naphthol) into its salt. These salts are soluble in water and therefore were located in the aqueous layers during the liquid-liquid extraction. These salts could then be acidified, which would return them from their salts to their original acid compounds. These acids are not soluble in water and therefore precipitate, which allows for easy recovery. Naphthalene, on the other hand, is a neutral organic compound, and therefore is not soluble in water or the aqueous solutions that will be produced throughout the experiment. It was located in the organic layer during the extraction process.
The
following flow chart shows the general procedure for isolating the
three
compounds (benzoic acid, 2-naphthol, and naphthalene) out of the
unknown
mixture using their solubility and that of their salts.
Formulas and
Structures of Organics: