 1.
1.Application of Scientific Concepts
Reflection
For application of scientific concepts I have chosen to use a project created in BIOlL 501. We were asked to design a web page that would cut across science curriculums. I chose to include aspects of biology and chemistry. The first part of this assignment was to create a background information piece on a chosen topic. It was to be written at a college level. In this paper I explain what happens biologically to cause the changing colors of leaves and the chemistry behind this process. The second piece of the work was a web page designed for young middle school students. Although I have used this with students, if I were to redo this assignment I would include some aspects of environmental science. I would include activities that would incorporate the environmental benefits of the falling leaves.
How this shows growth:
Prior to this paper I only knew that in order to survive during the winter the trees had to loose their leaves. It had something to do with the trees having to conserve energy. In this paper I learned the chemistry and biology behind how deciduous leaves change colors and why this occurs. In biology class we learned about the organelles that made up plant cells including the chloroplasts. In chemistry we learned about the electromagnetic spectrum and how objects absorb colors. This web page shows the application of these science concepts to a real world situation: how leaves change colors.
Evidence Below:
The Changing Colors of Fall
 1.
1.
Red, Yellow, and Orange colored leaves splash across the fall landscape in the Northeast corridor of the United States, a visual banquet that is breathtakingly beautiful. Colorful leaves shimmer in the sunlight, delighting our eyes with their beauty without taking into account the chemistry behind the vision. In order for this spectacular display to occur Mother Nature has to be working in her lab.
Why do the leaves of deciduous trees change colors? What is the chemistry behind the display? The answer is pigments. The pigments primarily responsible for the color of leaves are the following:
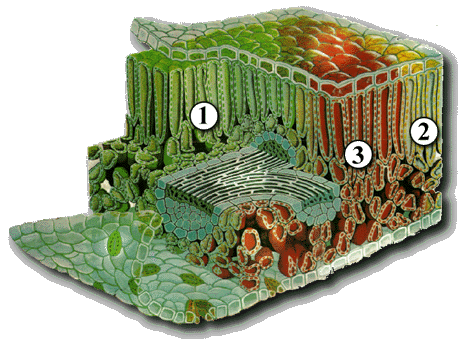
In winter the days are short and the temperatures are cold. The trees are holding on to the nutrients they gathered from the past growing season. As the days get longer, the dormant buds, little specks of green, yellow or dull red get ready to go about their job of producing new leaves that will provide the nutrients for the trees next growing season. The trees flower and new leaves develop and mature. The stored nutrients from last season are used for this process. By mid July they have reached their peak. Green envelops our eyes. Why, because of the chlorophyll pigments contained in the thylakoid compartment of chloroplast. These chlorophyll pigments are the glight catchersh or genergy thievesh depending on your perspective, but I prefer the former, since they are the driving force for photosynthesis and produce the products, carbohydrates, C6H12O6 and water, which are needed to sustain life.
So back to the point, why do we see green? Because light that is traveling to earth sends it in little packets called photons and these photon bundles carry different amounts of energy. Some of this energy can be seen in the form of visible light, red, orange, yellow, green, blue, indigo and violet but we donft always see all of it because it is absorbed by other objects. This is what happens in the leaf. The chlorophyll only needs certain colors to do its job, the other colors reflect back to our eye. The amazing thing about these photons is that they will be absorbed gonly if its energy is exactly what is needed to take the molecule from one allowed state to another.h (http://antoine.frostburg.edu/chem/senese/101/features/water2wine.shtml)
@
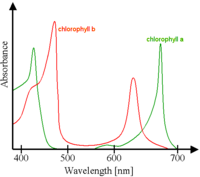
Each of the chlorophyll pigments will absorb specific colors from this spectrum sent by the sun. Chlorophyll gah predominantly absorbs the wave lengths Violet-Blue and Orange-Red. Chlorophyll gbh absorbs Blue-Green and Orange-Red wavelengths. The color not needed, green, reflects off and is received by the photoreceptor of our eyes, thus we see the color green.
Chlorophyll is a complex molecule containing a central magnesium atom surrounded with four nitrogen atoms from a large porphyrin ring and together with other pigments is the photoreceptor for the work needed to be done by the leaf. The actual structure of the molecule makes it insoluble in water. There are several variations but gah and gbh, are the pigments of photosynthesis. There is only a slight structural distinction; just enough to give them the minimally different absorption spectrafs mentioned above which causes the green color variation in leaves.
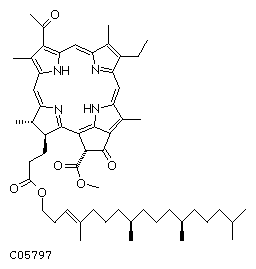
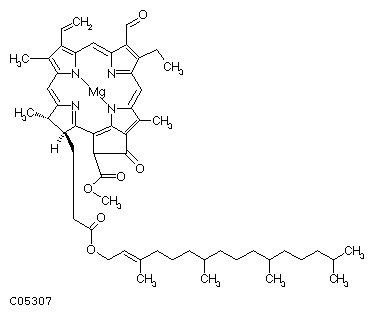
@
Chlorophyll a Chlorophyll b
C55H72O5N4Mg C55H70O6N4Mg
Chlorophyll gah is the most abundant in the leaves, making up about 75% of the plants chlorophyll pigment. It is also the only chlorophyll able to use the suns energy directly to convert solar energy to chemical energy. Chlorophyll gbh is the gaccessory pigment.h It absorbs the wavelengths that chlorophyll gah cannot take in immediately and then quickly passes them on to chlorophyll gah which uses them as if it received the light directly. gThe chlorophyll molecules are bound to specific integral membrane proteins along with carotenoids and electron transfer components necessary for photosynthesis. The proteins form a structured environment in which the chlorophylls absorb photons, but in which unwanted photodynamic reactions are relatively rare. The pigment-protein complexes are arranged into arrays of hundreds of pigment molecules called photosystems. Because of the spatial and geometric organization, most of the pigments function as light-harvesters and transfer the excitation energy to other pigments before unwanted photochemistry occurs.h (http://dwb.unl.edu/Teacher/NSF/C11/C11Content.html)
What does that mean? It means the chlorophylls are dangerous to themselves and they need their buddies the carotenoids to help protect them. How do they do this? Ifm so glad that you asked. Carotenoids are present in the spring buds but in small amounts. This changes as the season progresses and they are more needed as the sun becomes stronger. By the end of July they are at their maximum, working diligently behind the scene and waiting patiently to get an opportunity to show off.
@
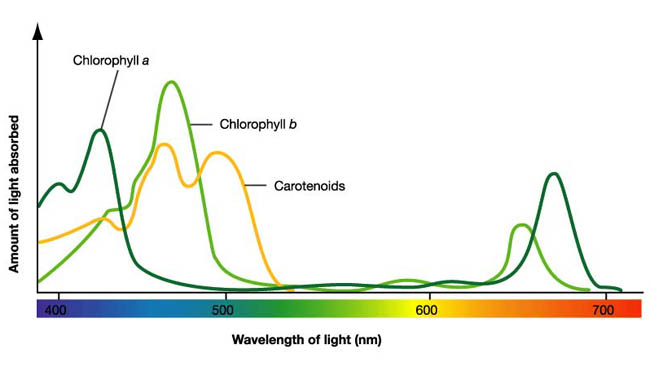
They belong to the family of compounds called gTetraterpenesh and occur in nature in two categories, carotene and xanthophylls. Carotenes contain carbon and hydrogen atoms. Xanthophylls (yellow leaves), have carbon, hydrogen and oxygen atoms. Their chemical structure causes them to be insoluble in water, like the chlorophylls. There are several that are present in all green leaves, beta carotenes and three xanthophylls: violaxanthin, antheraxanthin and zeaxanthin. Most leaves also contain alpha carotene and several other xanthophylls. They absorb photons in the blue region of the spectrum.
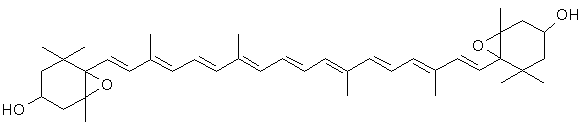 Violaxanthin
(
Violaxanthin
(C40H56O4)
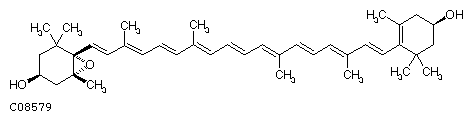
Antheraxanthin (C40H56O3)
@
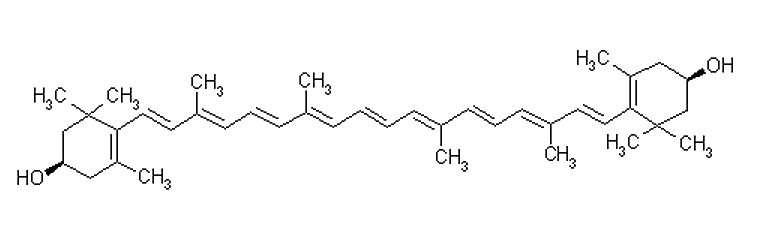
Zeaxanthin (C40H56O2)
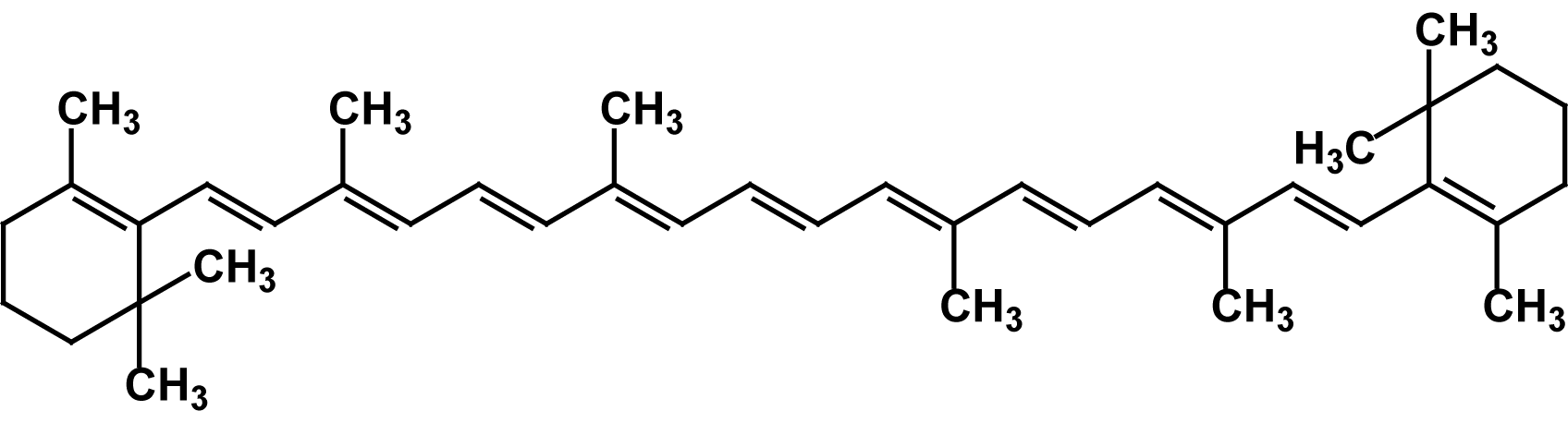 Beta-Carotene (
Beta-Carotene (C40H56)
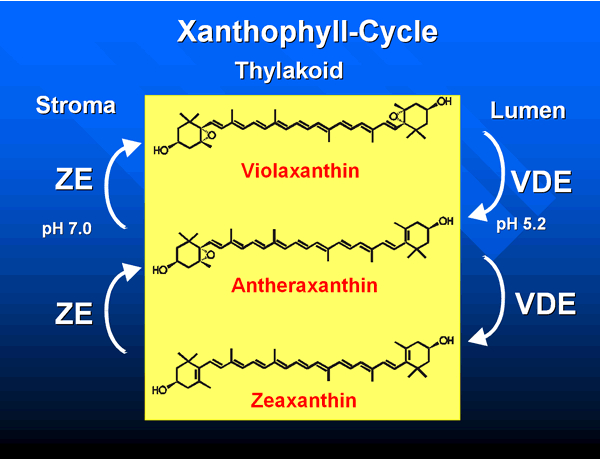
One of their functions, although not universal, is to assist, like chlorophyll gbh in transferring some of the energy collected to chlorophyll gah needed for photosynthesis. And another function, that has been hypothesized, is to protect the leaves from getting too much light. What! They also have to be careful of getting sunburn? Yes, because too much light can damage the molecules that make photosynthesis possible. Since the leaves, unlike us, are sessile, they canft move out of the way of the sun, so they use the gXanthophyll-Cycle.h It is believed that git involves reversible changes in the concentrations of the three yellow membrane pigments. In addition, Carotenoids have sometimes been implicated as the photoreceptors of light that prompt the plant to grow and develop. And thatfs not all, gOther suggested roles of the xanthophylls are protection against oxidative stress of lipids, modulation of membrane fluidity and regulation of abscisic acid synthesis.h (L.Cadova pg. 53)
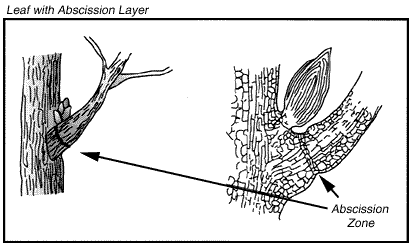
The longest day of the year varies from location to location. After this the days begin to get shorter little by little. At some point during this time even though we might not notice unless we were very careful observers, the trees begin to prepare for the dormant winter season. Scientists donft know exactly how the trees know what to do but the shorter days trigger the chemical reactions that take place. An abscission zone forms. A corky material below the zone begins to seal off the area that the leaf will detach from. gThe active summer leaves contain many valuable minerals and nutrients, and before the leaf is cut off from supplies these substances are substantially transported out of the leaves to avoid their loss.h (Prance, pg. 159)
The days are shorter, the light is less and the chlorophyll cannot continue to function properly under these conditions. It begins a steady decline, the Carotenoids also start to deteriorate but at a slower rate. First they get to show their stuff. What will we see? Look below. gBut wait!h you say. gWhy are they yellow and orange? And donft I see red!h Thatfs because this is not the end of the story! Take in the beauty and move on.
@
Yellow Poplar American Beech Sycamore Sugar Maple




@
Scarlet Oak Tulip Tree Sweet Gum
@



@
Sweet Gum

The third pigment responsible for the gorgeous display of colors is the water soluble anthocyanins, found in the class of Flavonoid pigments. They absorb green, blue-green and green light but unlike chlorophyll and Carotenoids, it is not found in all leaves, hence the color variations. Leaves with a large amount of anthocyanins and carotenoids both, will show orange. Leaves that have a lot more carotenoids but little or no anthocyanins will appear yellow. The trees with an abundance of anthocyanin will mask the carotenoids and appear red.
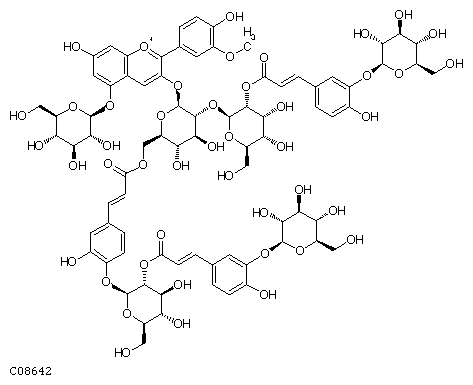
Anthocyanin ((C79H91O45)
So, how are these produced and why are some trees redder than others? As the chlorophylls and Carotenoids are busy breaking down the anthocyanins are busy producing. They are brought about by greactions between various sugars and complex compounds called anthocyanidins.h (http://www.agriculture.purdue.edu/fnr/html/faculty/Chaney/FallColor.pdf.) The colors can vary because there are a variety of them and they are influenced by the pH that is present. The more acidic, the redder the leaf; less acidity is responsible for showing more of a purple color. In addition, gTrees of the same species growing together often show a difference in color because of the individual variations in amounts of soluble carbohydrates.h (http://www.agriculture.purdue.edu/fnr/html/faculty/Chaney/FallColor.pdf.) Other factors that influence their production are fertile soil, light intensity, temperature and water supply. They like a lot of light so sunny days are a bonus. They react with the sugar, thus cool nights are helpful because the sugar in the leaves becomes more viscous. This is good because it traps the sap in the leaves which creates favorable conditions for the pigment to flourish. However, an early frost stops the production of the anthocyanins preventing them from showing their best colors as does wet and windy conditions which could cause the leaves to drop early.
Again, they are not just a pretty leaf. Their function is to act as a shield from the sunfs damaging rays protecting the delicate chemical reactions that are necessary while the leaf shuts down its food production for the winter and the tree extracts the nutrients from the leaves and deposits them into its buds, twigs, branches and roots before the leaf falls. It is also hypothesized that they might gincrease cold hardiness and drought resistance"(Lazarus et.al). Anthocyanins commonly appear during times of stress to a plant or tree. However, gthe major activity during leaf senescence is nutrient resorption for leaf production during the next growing season.h (Field) And finally gbecause anthocyanins are localized in the cell vacuole, they may be poised to scavenge oxygenated radicals leaking from chloroplasts as well as mitochondria and peroxisomesh (Field et.al) .
Finally the tree shuts down completely for the winter. The abscission controlled by the change in balance of ethylene and auxin has produced its protective layer of cork which shuts off any further leaf activity. The leaves break off and fall to the ground. A major reason for this is because there is less light and water available to the tree. Trees have to have water to draw in nutrients. Frozen water cannot be accessed, so to prevent excess drying during the winter, when water lost from the leaves cannot be replaced, the tree hibernates. The tree embraces the nutrients that will carry it through the winter and get it started in the spring. We look forward to reliving the cycle again but the next time you are enjoying the colors of fall, you will know that a lot of work went into creating the visual show that brings beauty to your eyes. Appreciate! Enjoy! And remember there is chemistry involved in the display!
Bibliography
· C. Diaz, V. Saliba-Colombani, O. Loudet, P. Belluomo, L. Moreau, F. Daniel-Vedele, J.-F. Morot-Gaudry, and C. Masclaux-Daubresse. gLeaf Yellowing and Anthocyanin Accumulation are Two Genetically Independent Strategies in Response to Nitrogen Limitation in Arabidopsis Thalianah Plant Cell Physiology., January 1, 2006; 47(1): 74 - 83.
· W. A. Hoch, E. L. Singsaas, and B. H. McCown gResorption Protection. Anthocyanins Facilitate Nutrient Recovery in Autumn by Shielding Leaves from Potentially Damaging Light Levels. Plant Physiology, November 1, 2003; 133(3): 1296 - 1305.
· Campbell, Neil A. and Reece, Jane B. Biology. CA: Pearson Education, Inc. 2002
· Faculty of Arts and Sciences of Harvard University. gLeaf Pigments.h Http://harvardforest.fas.harvard.edu/research/leaves/leaf_pigments.html
· Field, Taylor S., Lee, David W., and Holbrook N. Michele. gWhy Leaves Turn Red in Autumn. The Roll of Anthocyanins in Senescing Leaves of Red-Osier Dogwood.h Plant Physiology, October 2001; Vol. 127, pp 566-574
· Griffiths, Mary, Sistrom, W.r., Cohen-Bazie, Germaine, and Stanier, R.Y. gFunction of Carotenoids in Photosynthesis.h Nature, December 24, 1955. 176: 1211-1215.
· Helmenstine, Ann Marie. gChemistry of Autumn Leaf Color.h New York Times on the Web, 2006. http://chemistry.about.com/library/weekly/aa082602a.htm
· Howis, Seranne. gIntermediates of the Xanthophyll Cycle,h Rhodes University, Department of Botony, Grahamstown. http://rucus.ru.ac.za/wolfman/Essays/Xanth.html
· Krogh, David. Biology. New York: Pearson Education, Inc., 2005
· Lazarus, B.E., Schaberg, P.G., De Hayes, D.H. and Hawley, G.J. gRed Spruce Injury Winter Injury.h 2003 Canadian Journal of Forest Research. http://www.fs.fed.us/ne/burlington/research
· Lear, Brad. gAutumn Leaves,h ChemMatters, October 1986, pp7-10
· May, Paul. gChlorophyllh School of Chemistry, University of Bristol. http://www/chmbris.ac.uk/motm/chlorophyll/chlorophyll_h.htm
· National Science Foundation. http://dwb.unl.edu/Teacher/NSF/C11/C11Content.html
· Prance, Ghillean Tolmie. gLeavesh Crown Publishers, Inc., New York. 1985
· Sanger, Jon E. gQuantitative Investigations of Leaf Pigments From Their Inception in Buds Through Autumn Coloration to Decomposition in Falling Leaves.h Ecology:Vol.52, No6, pp. 1075-1089
· gScience of Color in Autumn Leaves.h United States National Arboretum. (http://www.usna.usda.gov/PhotoGallery/FallFoliage/ScienceFallColor.html
· Science Made Simple, gAutumn Leaf Color, Why Do Leaves Change Color in the Fall?h 2005. http://www.sciencemadesimple.com/leaves.html
· Senese, Fred. gThe Molecular Basis of Indicator Color Changes.h 1997-2005. Revised 9/20/2005. http://antoine.frostburg.edu/chem/senese/101/features/water2wine.shtml
· Tanino, Karen. gLeaf Coloration,h http://www.gardenline.usask.ca/trees/fall.html
· White, Michael A. Deciduous Forests." BookRags. Retrieved 2 August 2006, from the World Wide Web. http://www.bookrags.com/sciences/biology/deciduous-forests-plsc-02.html
Picture Credits
--------------------------------------------------------------------------------------------------------------------------------------
Children's Web Page: Go to site below
http://web-cygnus.sas.upenn.edu/~patricam/bioproject/index.html
or
for more accurate viewing go to site below and click on number 19, this can only be accessed from the MISEP participants only web page.
http://www.sas.upenn.edu/PennSTI/participants/MISEP1.html (number 19)
Photographs
Patty McCarrin - September 30, 2007 Back to e-portfolio
@
@
@
@
@
@
@
@
@
@
@
@
@
@
@
@
@
@
@
@