


Home MCEP home
E-portfolio CHEM502
GAMESS
WORKSHOP:
The Energy Profile for the Formation of Formaldehyde to Hydroxycarbene
|
Purpose:
GAMESS and MacMolPlt software is unlike Chem3D's spring-and-ball,
classic mechanical calculations (MM2) because it calculates the
energies of a molecule's electrons using quantum mechanics (Schrodinger
equations, Hessians, etc.). The purpose of this exercise is to
become familiar with its use.
The ground states (optimized geometries) of structural isomers HCOH (a
carbene) and H2CO (formaldehyde, a
ketone) and the transition structure that connects them at the HF/3-21G
level of theory were calculated using GAMESS. The difference in
energy for the two isomers and the activation energy barrier (energy
difference with the transition state energy) was also calculated.
Energy Profile of Reaction and
Pictures of Geometries:
1.
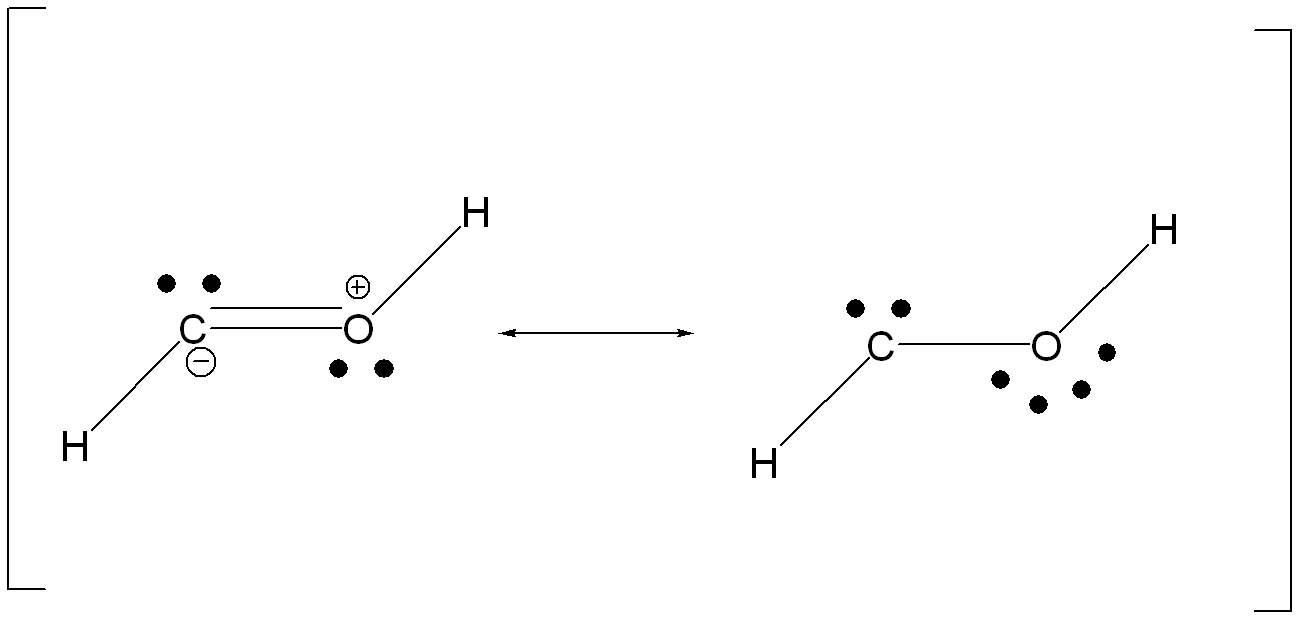
HYDROXYCARBENE
Energy: -113.1463 Hartrees = -71,000.43471 kcal/ mole
|
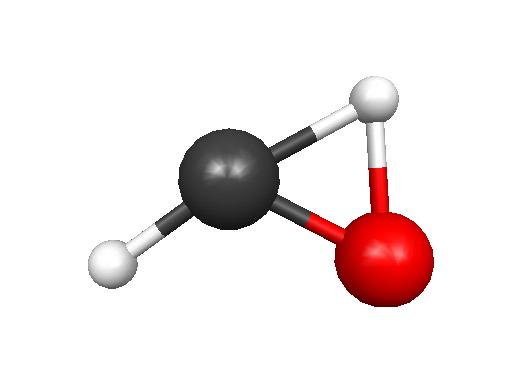
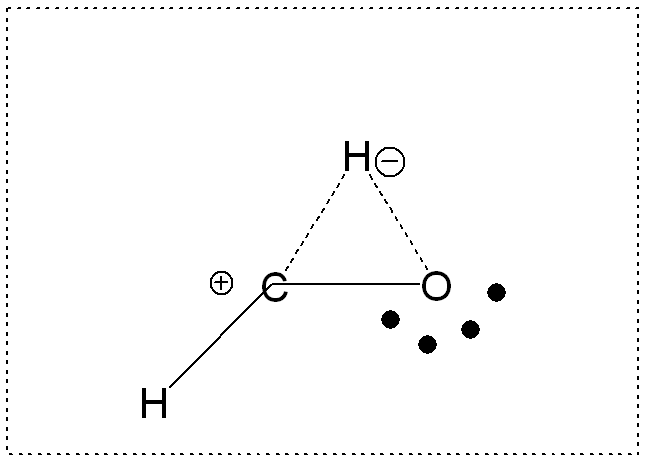
2. TRANSITION STATE
Energy: -113.0501 Hartrees = -70940.06825 kcal/ mole
|
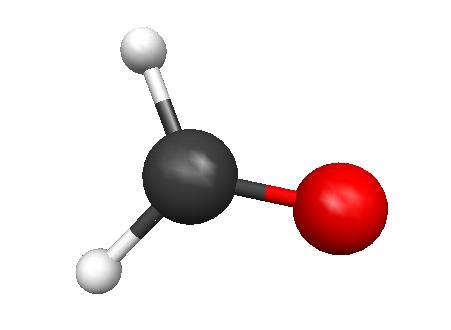
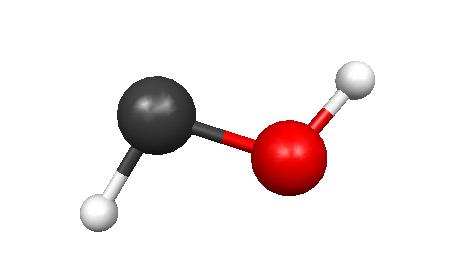
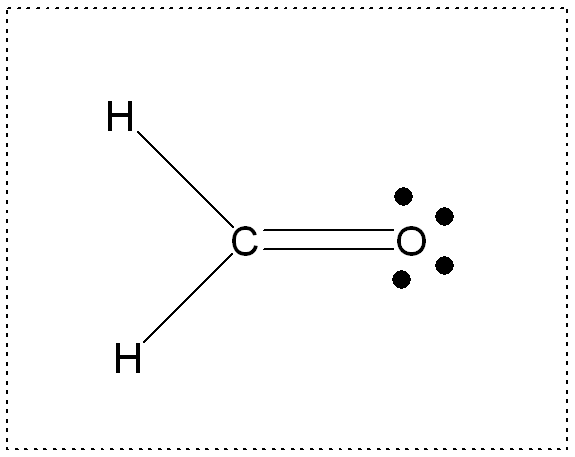
3.
FORMALDEHYDE
Energy: -113.2218 Hartrees = -71047.81172 kcal/ mole
|
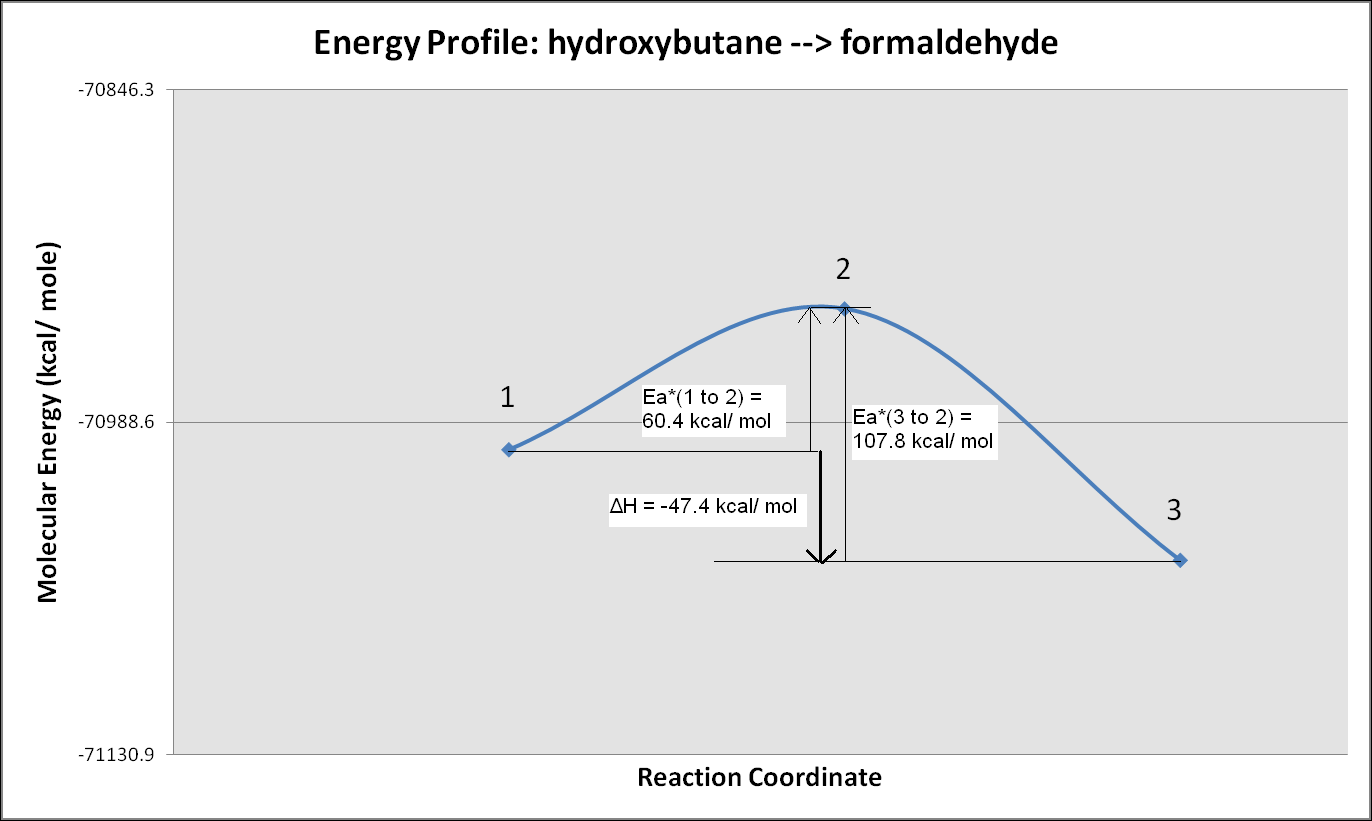
The
predicted difference in energy between the hydroxycarbene and the
formaldehyde isomers is -47.4 kcal/ mole as shown in the energy profile
graph. The activation energy barrier from hydroxycarbene to the
transition state is 60.4 kcal/ mol. The activation energy barrier
from formaldehyde to the transition state is 107.8 kcal/ mol.
An
analysis of the geometric structures and the lewis structures for the
two isomers and the transition state gives a good explanation for the
observed energies.
1)
The formaldehyde isomer is the lowest energy molecule--all molecules
have filled valence shells and zero formal charges.
2) The hydroxycarbene has greater energy
because, judging from its resonance structures, the highly
electronegative oxygen has a partial positive formal charge (indicating
a lower electron density than what is desirable) and the carbon is not
completely satisfied with a full octet--this leads to a highly
reactive, higher energy molecule.
3)
The transition state is the highest energy state where hydrogen is
pushed toward the carbon atom and is in the process of forming a bond
with carbon and breaking its bond with oxygen... leading to a highly
strained pseudo-ring structure. The formal charges in the Lewis
structure further represent the instability of the electron arrangement
in the transition state.







