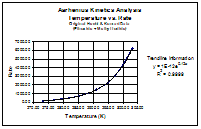
Temperature
vs. Rate
For the first graph, I plotted the Hecht & Konrad data as a Temperature vs. Rate relationship. This produced a fairly smooth exponential curve. When I applied a trendline, and found the trendline equation, the equation was found to be an exponential equation, in which e is raised to the power of x(fraction of a number). This reflects the Arrhenius equation (see equation above). The R2 value is .9999, indicating a high level of accuracy in the data points.
Given that ln(x) = ex, it would follow that if the data were viewed as 1/T vs. ln(rate), we would see a straight line, if Arrhenius' equation is valid.
Click Here to See the Graph and Data
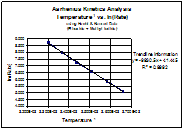
Temperature-1 vs. ln(rate)
In fact, we do see the predicted relationship. With further examination, we can also see that if we manipulate the Arrhenius equation to solve for Ea, the slope of our line will be equal to Ea.
k = Ae-Ea/RT
lnk = ln(Ae-Ea/RT)
lnk = lnA + lne-Ea/RT
lnk = lnA - Ea/RT
lnk = - Ea/R (1/T) + lnA
lnk = ln(Ae-Ea/RT)
lnk = lnA + lne-Ea/RT
lnk = lnA - Ea/RT
lnk = - Ea/R (1/T) + lnA
Graph #2
Click Here to See the Graph and Data