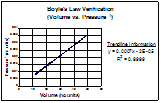
Volume vs.
Pressure
For the first graph I plotted Boyle's data as a volume vs. pressure relationship. This produced an exponential curve that suggests that as volume increases, pressure decreases. When I applied a trendline, the trendline equation, though exponential, showed an inverse relationship. This is so, because x is raised to the power of -.9984, which is almost -1. Any x raised to the power of -1 is equal to 1/x.
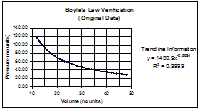
For this reason, I surmised that if I created a graph where the relationship was pressure vs. the inverse of temperature, I would have a straight line.
Pressure vs.
Volume-1
In fact, I was correct in my assumption. This graph shows a straight line that shows an inverse relationship between pressure and temperature. This is a clear affirmation of Boyle's Law, as stated above.
For additional confirmation I chose to graph the other inverse relationship.
Pressure vs. Volume-1
Click here for the graph and data
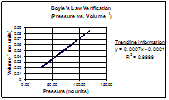
The other inverse relationship,
volume vs. the inverse of pressure, also showed the straight
line. This clearly establishes the accuracy of Boyle's Law.
Volume vs. Pressure-1
Click here for the graph and data