Contents
Raman Spectroscopy and the Analysis of Gemstones

 |
Contents Raman Spectroscopy and the Analysis of Gemstones |
 |
|
~ Raman Effect / Raman Scattering ~
Photons are capable of being scattered by a molecule’s electronic
system if the medium through which the photon travels is made up of
molecules that are capable of changing the path of the incident
photon. When photons pass through a transparent sample of matter,
most of them will continue in the same direction, however a small
fraction of them will be scattered in other directions. When a photon
interacts within a system, it can be scattered by one of three
different methods (7):
1) Rayleigh scattering 2) Raman -Stokes scattering 3) Raman -Anti-Stokes scattering Rayleigh scattering, named in honor of Lord Rayleigh who identified the radiation effect, is sometimes referred to as elastic scattering. This effect will occur when the radius of the scattering sphere is significantly smaller than the incident wavelength. (14) As such, the scattered photon retains its incident wavelength (as well as its frequency and energy). The majority of photons that are scattered upon interaction with a molecular system do so in this elastic manner. (1) However, sometimes when photons are scattered due to the vibrations of molecules present in a transparent medium, there is a shift in wavelength compared to that of the incident wavelength. Occurrence of such a shift has been termed the Raman effect (named after C.V. Raman, the physicist from India who discovered the effect). The wavelength shift characterizing Raman scattering can be as great as 4000 cm-1. (15) As shown in the equation below, the degree of Raman shift is dependent upon the mass of the atoms of a particular covalent bond, and the strength of the bond. 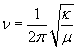 where κ is a measure of
bond strength, and μ is the reduced mass. (2)
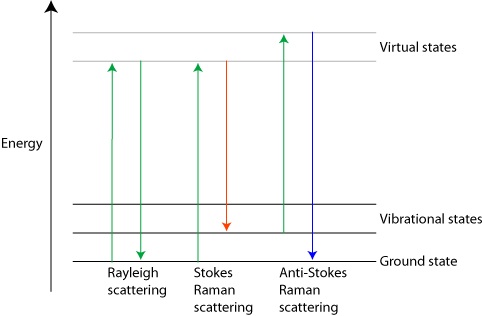
Two different forms of Raman scattering are possible since the light photons that are scattered can either gain or lose energy compared to the incident energy. If a molecule that begins in its vibrational ground state is excited to a virtual state (resulting from polarization of the electron cloud) upon absorption of an incident photon, and returns to a higher energy vibrational state, then the scattered photon emitted has less energy (longer wavelength) than the incident photon. The excess energy has been gained by the scattering medium. This type of Raman effect is called Stokes scattering. (6) On the other hand, if the molecule begins in a higher vibrational state and is raised to a higher energy virtual state upon absorption of a photon, the return of this molecule to the ground vibrational state will be characterized by the emission of a photon of higher energy (shorter wavelength) than that of the incident photon. As such, energy has been lost by the scattering medium. This type of Raman effect is called anti-Stokes scattering. (6) Table 1, below, shows a comparison between the main concepts of Raleigh scattering and Raman scattering. In addition, Figure 1 shows a comparison of energy diagrams for each of these types of scattering. Table 1 - Comparison of Raleigh and Raman
Scattering
Figure 1 - Energy diagrams for Raleigh and
Raman Scattering (6)
 The Raman effect is a relatively weak effect, in comparison to the Rayleigh effect. Less than 1% of scattered photons demonstrate the Raman effect compared to over 99% which exhibit the Rayleigh effect (4). Of those photons that are Raman scattered, the amount of Stokes scattering is greater than the amount of anti-Stokes scattering. This is expressed in the difference in intensity of Stokes and anti-Stokes scattering (see Figure 2 below). This can be explained by the differences in population of the vibrational states of a molecule, which follows the Boltzmann distribution. According to this distribution, there would be fewer molecules that start out in a higher vibrational state compared to the ground vibrational state, and thus fewer molecules that would emit photons characteristic of anti-Stokes scattering compared to Stokes scattering. (6) Figure 2: Varying
intensities for the three forms of photon scattering. (6)
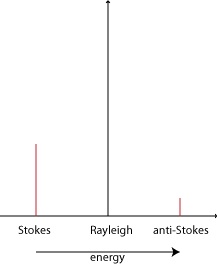 Shifting due to the Raman effect is due to the spacing between vibrational states and the ground state. As such, the Stokes and anti-Stokes photons will be scattered an equal amount of energy (below and above, respectively) from the Rayleigh photon energy. (6) |
|||||||||||||||