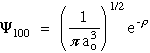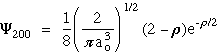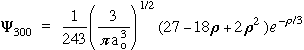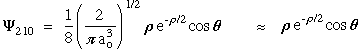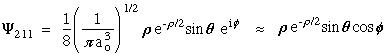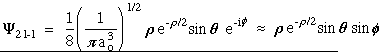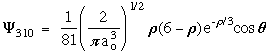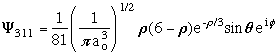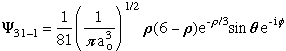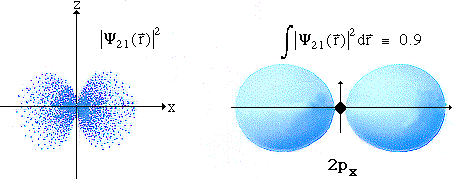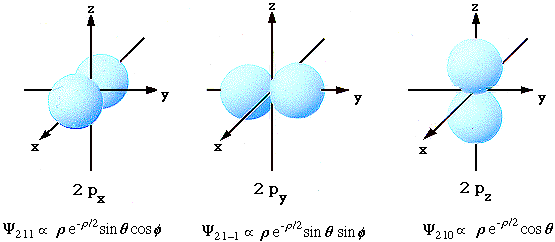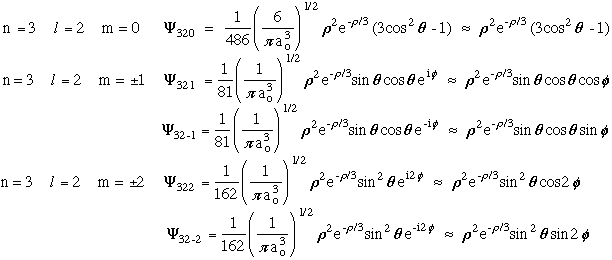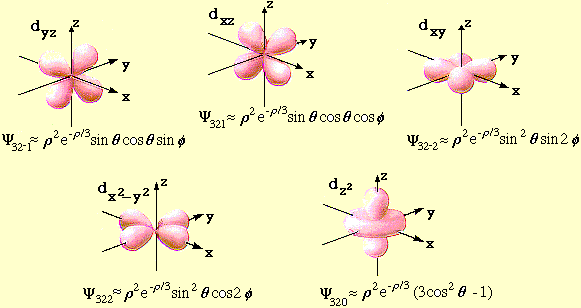III. Quantum Mechanics
Figure 2.5.1:
Heisenberg "Uncertainty Principle" Experiment
- A comparison of Maple and Mathematica . (Mathematica
to be used for solving the Schrodinger Equation for the "Particle-in-a-1D-Box"):
Maple input: >deqPB: =diff (y(x),x,x)=-E* y(x);
Maple output:
Mathematica files:
- Solving
the Schrodinger Equation for the "Particle-in-a-Box"
"Particle-in-a-Box" Wave-Functions
1-D and 2-D
- Orbitals for Hydrogen-Atom, e-
Spherical Coordinates: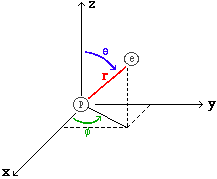
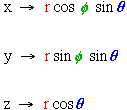
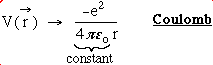
Rewriting the Schrodinger equation in spherical coordinates:

where 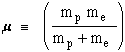 and
and .
.
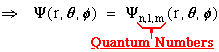
The EXACT solution!!:
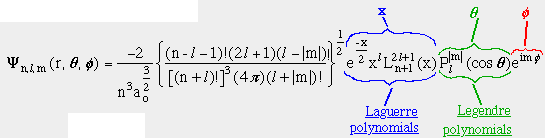
where 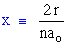
this implies: n, l, m, the quantum numbers, completely specify
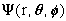 .
.
Orbital Size: n: principal quantum number (n=1,2,3,...)
Orbital Shape: l: angular momentum quantum number [l = 0,1,2,...(n-1)]
Orbital Direction: m: magnetic quantum number (m = 0, +-1, +-2, ...+-l)
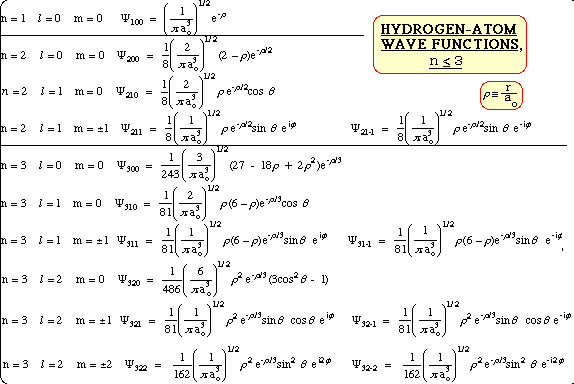
- Hydrogen Atom Wave Functions
note: energy-eigenvalues are functions of n only!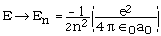
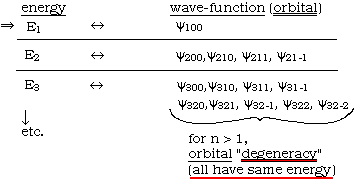
Meaning of 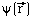 via
via 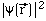 :
:
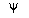 is a complex number, i.e.,
is a complex number, i.e., 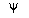 = a + bi
= a + bi
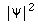 is a real number, i.e.,
is a real number, i.e., 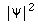 =
= 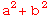 = (a+bi)(a-bi)
= (a+bi)(a-bi)
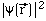 : "probability density distribution"
for the electron, i.e., probability /
: "probability density distribution"
for the electron, i.e., probability / 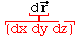 vs. electron position
vs. electron position
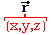 (vector)
(vector)
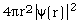 : "radial probability density distribution"
for the electron, i.e., probability/dr vs. electron radial position
: "radial probability density distribution"
for the electron, i.e., probability/dr vs. electron radial position
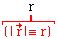 .
.
Figure
2.12.1: The Hydrogen 1s Orbital (probability density distribution)
Figure 2.12.2: The Hydrogen
1s Orbital (radial probability distribution)
- l=0 Hydrogen Atom Wave Functions
- n = 1 l = 0 m = 0
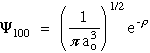
n = 2 l = 0 m = 0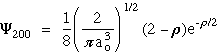
n = 3 l = 0 m = 0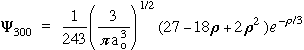
- l = 0 Probability Density Plots and Cloud Pictures
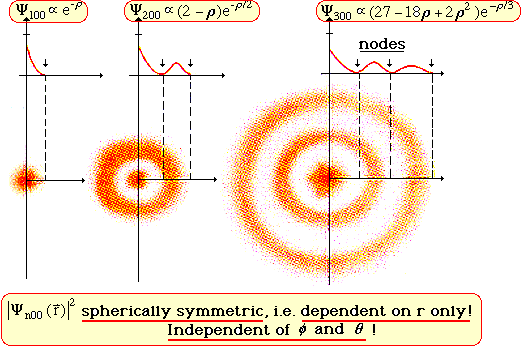
Mathematica files:
- l=0 Hydrogen Atom Wave
Functions
l=0 H-Atom Wave Functions
Set Up of Contour Plots
l=0 H-Atom Wave Functions Contour
Plots in 3-D
- l=1 Hydrogen Atom Wave Functions
n = 2 l = 1 m = 0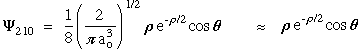
n = 2 l = 1 m = +-1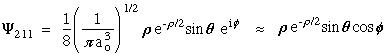
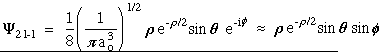
n = 3 l = 1 m = 0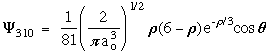
n = 3 l = 1 m = +-1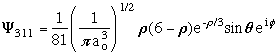
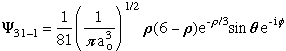
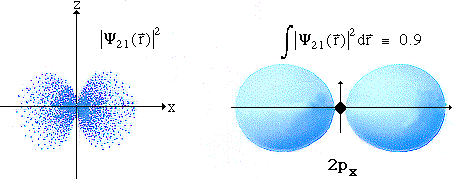
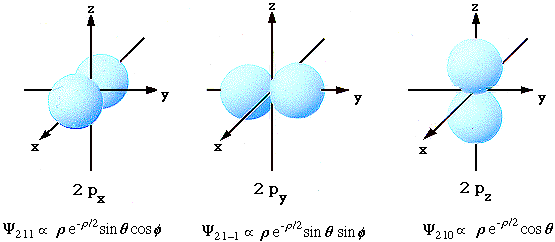
Mathematica files:
- l = 1 H-AtomWave
Functions
- l = 1 H-Atom Wave Functions Contour
Plots in 3-D
- l = 2 Hydrogen Atom Wave Functions
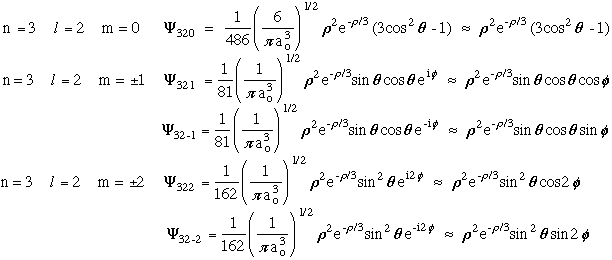
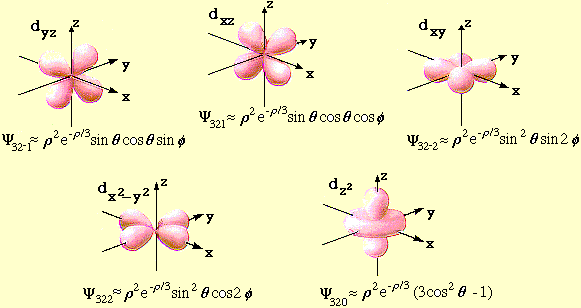
Mathematica files:
- l = 2 H-Atom Wave
Functions
l = 2 H-Atom Wave Functions Contour
Plots
You can now go to the Topics to be Covered
page or the unit outline.


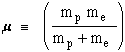 and
and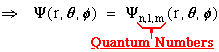
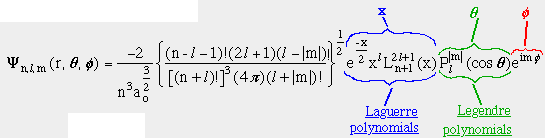


![]() is a real number, i.e.,
is a real number, i.e., ![]() =
= ![]() = (a+bi)(a-bi)
= (a+bi)(a-bi)