
| Home | UPenn E-Portfolio | CHEM
501 |
CHEM 502 |
CHEM 503 |
EDU 536 |
CHEM 505 |
CHEM
506 |
CHEM 507 |
EDU 636 |
CHEM 504 |
CHEM 508 |
| Reflection
#8 Use of New Pedagogical Knowledge in Designing Instruction |
"The participant has demonstrated the application of new scientific
knowledge in the design of educational theory in the design of teaching
materials of lessons used in his or her own classroom."
| <--Previous Reflection Next Reflection--> |
Related Courses:
- Chemistry 501-General and Organic Chemistry I
- Chemistry 506-Inorganic Chemistry
- Chemistry 507-Molecular Spectroscopy
I have chosen to include past and present lesson plans to show growth in the area of using new pedagogical knowledge in designing instruction. My Baseline Evidence 1 includes a lesson plan from my UPenn STI application. This evidence shows my initial understanding of how to effectively design instruction. My Baseline Evidence 2 includes a lesson plan from my high school classroom on how I have taught phase diagrams, including a visual powerpoint. This evidence also shows my initial understanding of how to effectively design instruction. The Later Evidence 1 is a lesson plan two years after the baseline lesson plan from my high school classroom. The instruction design has changed from lecture to having students working in groups and answering questions together. The Later Evidence 2 includes a Process Oriented Guided Inquiry Learning (POGIL) model that I have used to design the instruction by asking critical thinking questions. The POGIL created is to show how questions were asked to promote critical thinking. Lastly, the Later Evidence 3 I have included is a centralized website (from Inorganic Chemistry) which contains other websites on four topics: matter, VSEPR theory, stoichiometry, and laboratory safety. This website has been used to design instruction, incorporating technology and student guided learning. Students could search out further information on the lesson by going to these websites.
How:
The evidence of the lesson plans, created two years apart, shows the growth that I have made in incorporating a new design of instruction in my classroom. The Baseline Evidence 1 of a lesson plan uses a demonstration, a video from a website, and teacher created notes to guide the content of the instruction. Lecture notes and practice problems are used in designing the instruction. In the Later evidence 1 & 2, a change was made to incorporate content in the form of a POGIL. This guided inquiry learning was a change in practice for my classroom because the focus shifted from teacher-directed to student-directed learning. Students were guided through a series of increasingly difficult questions to arrive at an understanding of phase diagrams. This designed instruction shows how, with careful questioning techniques, students can gain the understanding of content (phase diagrams) without lecture. Before UPenn's Science Teacher Institute I would have never attempted to create a POGIL nor seen the need to give my students this learning tool. Lecture would have been my preferred method of carrying out my instruction. The Later Evidence 3 relates to the rubric item because it provides a practical place where students from my class can go to gain content information. I have provided a place where students can go to obtain more information on the topic of phase diagrams (matter websites). I can now use this website to design instruction future instruction on various topics
Why:
I choose these pieces of evidence to show growth in the area of using a new design of instruction. The lesson plans (Baseline Evidence), written two years apart, show the differences in my approach to a particular topic, phase diagrams. The POGIL (Later Evidence 2) shows that I can take content commonly found in lecture style and convert them to design my own unique instruction that is more effective for the learner. The students loved the POGILS and were challenged by the increase in inquiry-based thinking. I first learned of this constructivist inquiry-based approach in the summer of 2005. I met Rich Moog, (director of the POGIL movement) and attended a workshop to understand how to create POGILs and the learn the methodology behind the types of questions asked. According to Spencer (1999), one of the leaders of the POGIL movement, the constructivist-learning-cycle approach (also called “inquiry-based”) "has been shown to facilitate retention of information and the transfer of thinking skills and content.” The constructivist learning cycle allows learners to construct their own knowledge in the classroom. The student is not merely the recipient of knowledge but the creator of knowledge through carefully guided inquiry experiences (Spencer, 1999). In addition, this approach is usually conducted within a community of learners. I firmly believe in this inquiry-based approach, which is reflected in the assignments I have given to my students. One assignment is the phase diagram POGIL (Later Evidence 2). I used this assignment to construct thier ideas concerning phase diagrams andassess student understanding of phase diagrams. According to Spencer (1999), inquiry based thinking has a positive effect on students' ability to construct their own knowledge. I was doing less work, and the students were doing more thinking. A recent professional development session confirmed a conviction I had based on the first summer in the MCEP program: “The one who does the work, does the learning.” The shift in my instruction has affected how I approach my class on a daily basis. Students are given more responsibility for their own learning.
Baseline Evidence 1:
| From Submitted
Lesson Plans, UPenn STI Application, 2005 Introduction:
|
Baseline Evidence 2:
| From Submitted
Lesson Plans, Mastery Charter High School, 2005 In my chemistry class I would use basic power point slides to lecture about what a phase diagram is. Here are the actual lesson plan objectives: "Given Lecture Notes on Phase Diagrams in bulk pack and diagram pictures, SWBAT determine which section is which and determine which process is associated a change in temperature/pressure." "Given information on Phase diagrams, SWBAT complete a practice problem of drawing their own phase diagram on a piece of paper to 75%." |
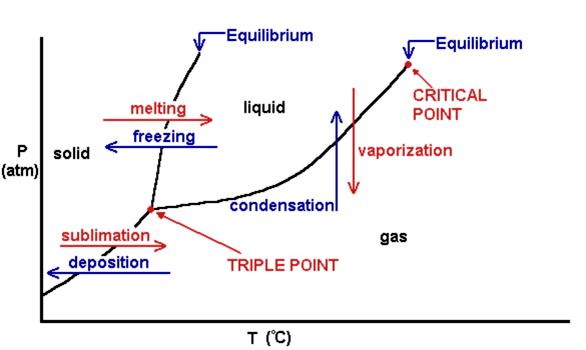 Phase Diagram
PowerPoint Slide Used To Lecture
|
Later Evidence 1:
| From Submitted
Lesson Plans, Mastery Charter High School, 2007 Here are the actual lesson plan objectives: "Given Brief introduction on Phase Diagrams in bulk pack and diagram pictures, SWBAT complete Phase Diagram POGIL in groups and answer the questions that are included." "Given information on Phase diagrams, SWBAT complete a practice problem of drawing their own phase diagram on a piece of paper to 75%." |
 Phase Diagram PowerPoint Slide Used in POGIL |
Later Evidence 2:
| Text from Phase
Diagram POGIL, Created for Mastery Charter High School, 2006 INFORMATION: Critical Thinking
Questions What Label is on the x-axis?
|
Later Evidence 3:
| From Chemistry
Websites, Chemistry 506, 2007 "The following chemistry website topics are organized below. Each topic contains websites with a brief descriptions for teachers and/or students." |
References
J. N. Spencer, "New Directions in Teaching Chemistry: A Philosophical and Pedagogical Basis." J. Chem. Educ., 1999, 76, 566-569.