Atomic
Absorption Spectroscopy

The begining of Atomic Absorption spectroscopy
began with the chemist William Hyde Wollaston when he first absorbed
absorption spectra of solar rays. Later, Gustav Kirchhoff
determined that the spectra lines were from the absorption of vapor's
in the sun's atomosphere. Talbot an Herschel laid the ground work
for identifying compounds when they observed that a flames color would
change when different compound salts were exposed to the flame.
Alan Walsh (pictured left) is credited for the establishment of the
Atomic Absorption principle between 1952 and 1977 while working
for the CSIR Division of Industrial Chemistry in Melbourne,
Australia. As we all have surely done on a Sunday
afternoon, Alan began to wonder why Molecular spectra was found in
absorption and Atomic Spectra in emission. He began to think that
the Absorption spectra had much more to offer. He also thought
that since the thermal source was more easily controlled there would be
less inter-element interference then was currently being experienced in
emission spectroscopy. In his first experiments he used a sodium
vapor lamp and a direct vision spectrometer. The intensity was
measured by a photomultiplier tube and recorded by a cathode ray
oscillograph. He sprayed sodium chloride solution over the simple
flame and established the basis for atomic absorption
spectroscopy. He had difficuly reproducing the same resultd for
other elements however and realized he needed a much stronger light
source. He came to the result of using hollow cathode
lamps. Alan Wlash had done it! He developed the first
atomic absorption spectrometer that had all the components now included
in every AASpectrometer today! They were a sealed hollow cathode
lamp as the source, flame atomizer as the absorber, and a tuned
amplifier. For the full biographpy and development of AAS by
walsh and his collegues please visit http://www.science.org.au/academy/memoirs/walsh2.htm#11.
Atomic Absorption
spectroscopy is specifically used to determine the concentration of a
metal in a substance. The
concentrations are usually low and measured in mg/L. AAS measures
the absorption of light of the
atoms of a substance in the gas phase.  Therefore substance must
first be dissolved in a liquid, dried and then atomized to vaporize the
substance into gas atoms.
Therefore substance must
first be dissolved in a liquid, dried and then atomized to vaporize the
substance into gas atoms. 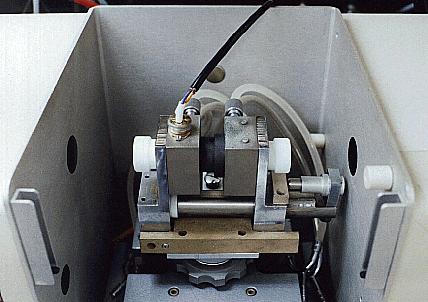 Please
refer to the Sample Preparation page for more details on how toys are
prepared for analysis. There are two
types of AAS equipment, Flame AAS and Graphite Furnace AAS. (pictured
to the left anf right respectivly) Flame AAS as the name states
uses a flame to directly heat the sample and break it down into its gas
phase. Graphite Furnace AAS is generally electrically powered to
heat a graphite column where the sample has been injected. The
graphite furnace AAS is generally more efficent, it can accept both
liquid and organic samples and also in very small amounts. The
liquid samples are dried to ash and then atomized and the organic
samples are reduced to ash and then atomized.
Please
refer to the Sample Preparation page for more details on how toys are
prepared for analysis. There are two
types of AAS equipment, Flame AAS and Graphite Furnace AAS. (pictured
to the left anf right respectivly) Flame AAS as the name states
uses a flame to directly heat the sample and break it down into its gas
phase. Graphite Furnace AAS is generally electrically powered to
heat a graphite column where the sample has been injected. The
graphite furnace AAS is generally more efficent, it can accept both
liquid and organic samples and also in very small amounts. The
liquid samples are dried to ash and then atomized and the organic
samples are reduced to ash and then atomized.
Once the sample has been atomized it is exposed to
ultraviolet or visible light to determine the transitions of energy
levels. The light is absorbed by the electrons and moves them
from their ground state to their excited state. Transitional
energies which are specific to compounds and elements lead to their
identification are then directed to the detector where readings are
recorded. 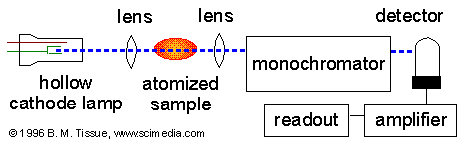
Callibration of the spectrometer is of the utmost importance
before these readings are taken. Solutions
of known concentrations should be run to establish a calibration curve
which is compared to the unknowns and consequently identified.
The amount of light the sample finally absorbs will determine its
concentration; calculations are made using the concepts of the
Beer-Lambert Law.
The Beer-Lambert Law is a
logarithmic relation between the transmission (T) of light through the
sample, the absorption coefficent (α) of the sample and the path
length (ℓ).

where I0 and I are the
intensity of the incident light and the sample after energy has been
absorbed.
References
http://www.spectroscopynow.com/coi/cda/detail.cda?page=2&id=1905&type=EducationFeature&chId=1
http://www.galbraith.com/spectroscopy.html
http://www.scribd.com/doc/10514011/Atomic-Absorption-Spectroscopy


 Therefore substance must
first be dissolved in a liquid, dried and then atomized to vaporize the
substance into gas atoms.
Therefore substance must
first be dissolved in a liquid, dried and then atomized to vaporize the
substance into gas atoms.  Please
refer to the Sample Preparation page for more details on how toys are
prepared for analysis. There are two
types of AAS equipment, Flame AAS and Graphite Furnace AAS. (pictured
to the left anf right respectivly) Flame AAS as the name states
uses a flame to directly heat the sample and break it down into its gas
phase. Graphite Furnace AAS is generally electrically powered to
heat a graphite column where the sample has been injected. The
graphite furnace AAS is generally more efficent, it can accept both
liquid and organic samples and also in very small amounts. The
liquid samples are dried to ash and then atomized and the organic
samples are reduced to ash and then atomized.
Please
refer to the Sample Preparation page for more details on how toys are
prepared for analysis. There are two
types of AAS equipment, Flame AAS and Graphite Furnace AAS. (pictured
to the left anf right respectivly) Flame AAS as the name states
uses a flame to directly heat the sample and break it down into its gas
phase. Graphite Furnace AAS is generally electrically powered to
heat a graphite column where the sample has been injected. The
graphite furnace AAS is generally more efficent, it can accept both
liquid and organic samples and also in very small amounts. The
liquid samples are dried to ash and then atomized and the organic
samples are reduced to ash and then atomized. 
