


| BADoyle | Chem 501 | Chem 502 | E-Portfolio |
| Chem 503 |
Educ
536 |
Spectroscopy | Educ 636 |
 Barbara
A. Doyle
Barbara
A. Doyle
Lead is one
of the most dangerous
heavy metals. It is especially toxic to children damaging neuronal
connections,
causing brain and blood disorders. Lead in the body inhibits cell
function and can cause damage to kidneys, nervous system and red blood
cells. People with high levels of lead in their system can suffer
from anemia, hearing loss, brain swelling, convulsions, coma and even
death. Children are at the highest risk since they are the ones playing
with the tainted toys and infants and toddlers are even more at risk
since the tend to place everything in their mouths. Symptoms that
would show evidence of lead poisoning in children would be fatigue,
headaches, stomach aches, muscle pain and behavioral problems.
Children may even display changes in their school performance and slow
growth.
Phthalates
are esters that are primarily used as plasticizers. A Plasticizer
is a substance that is added to a plastic to increase its flexability.
high doses have been shown to change hormone levels, cause birth
defects, and damage liver and testes. Phthalates have also been
connected to higher percentages of allergies and asthma in
children. Infants are at a great risk for exposure since most
teething rings and toys contain phthalates to make the plastics more
malleable.
What is Spectroscopy?
People have been studying light since the begining of time, earliest
mention of these studies are about the refraction of light.
Ptolemy in 139 AD created tables on the reflection and refraction of
light. Theodoric of Freiberg in 1304 explained the refration of
light in rain drops to create rainbows. Royen around 1620
discovered the law of refraction and in 1752 Melvill observed the
first line spectrum. In 1800 Hershal discovered Infrared, and the
19th century brought the discovery of ultraviolet light and the
principles of interference of light. Wave theory to explain
diffraction was developed by Schwerd in 1835. The first
spectrometer was created in 1851 and emission lines of iron and copper
were recorded. Developments continued to progrss through the
centuries to brig us to today's current methods (. Specific
histories will be incorporated on the XRF and AAS pages.
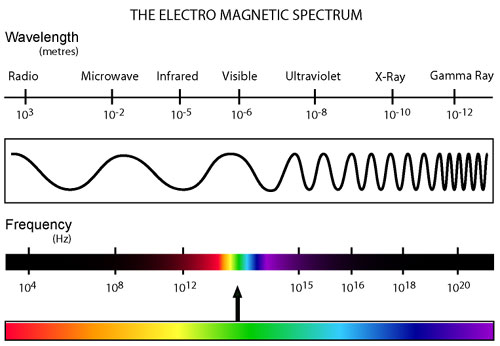 Spectroscopy
is the study of light, the electromagnetic spectrum (pictured
left) with its wide range
of wavelengths and its
interactions with matter.
Spectroscopy
is the study of light, the electromagnetic spectrum (pictured
left) with its wide range
of wavelengths and its
interactions with matter. 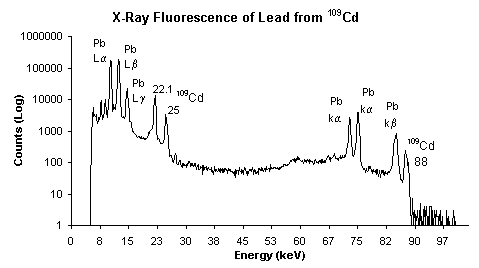 Spectrometers measure the dispersion of light according to its
wavelength, frequency and energy. The
dispersion is generally caused by a prism that is part of the
spectrometer. The
spectrometer reads the emissions of this spectrum of diffracted light; these
emissions then correspond to specific elements.
The collected data is then displayed as a function of intensity vs.
energy, an example of a lead spectrograph using X-ray fluorescence is
pictured right.
Spectrometers measure the dispersion of light according to its
wavelength, frequency and energy. The
dispersion is generally caused by a prism that is part of the
spectrometer. The
spectrometer reads the emissions of this spectrum of diffracted light; these
emissions then correspond to specific elements.
The collected data is then displayed as a function of intensity vs.
energy, an example of a lead spectrograph using X-ray fluorescence is
pictured right. 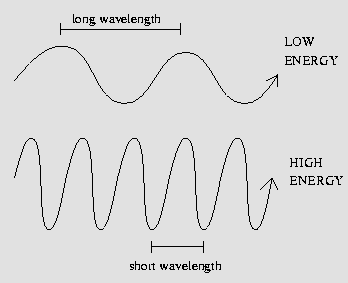
The energy from a photon is inversely
related to its wavelength by the equation, E = (hc)/λ. E
is the energy in photons measured in Joules, h is
plank's constant, c is the speed of light, and λ is the
wavelength in nanometers. An
inversely proportional relationship means the shorter the wavelength
the higher the frequency and vice versa. (pictured right) The
energy of the incident light specifically excites the electrons of the
atoms and takes them from their ground state to their excited
state. The energy associated with this change in state is the
atoms ionization energy which is specific to each element. As
electrons fall back into the gaps created by these shifts light is
emitted that reveals the ionization energies that identify the elements
in the compounds. This is how Emission spectroscopy works.
Absorption spectroscopy is the opposite, light is passed through a
prism and through the sample, the emitted wavelengths are the ones not
absorbed by the sample. The elements in the sample can be
identified by these missing wavelengths.
http://www.files.chem.vt.edu/chem-ed/spec/spectros.html
| Spectroscopy Home |
Toy Safety Standards |
Sample Preparation |
| XRF |
AAS |
Lesson Plan |