WORKS CITED:
- Maseka, K. (2005). The emission and absorption of radiation
by white and dyed cotton fabrics laundered with fluorescent brightening
agents. AATCC Review, 5(10), 35-38.
- Image of
fluorescing tonic water from Wikipedia Commons. http://en.wikipedia.org/wiki/File:Tonic_water_uv.jpg
(April 2, 2009).
- Image of additive
color mixing. http://www.d.umn.edu/~mharvey/th1501color.html
(January 7, 2009).
- Image of
electronic and vibrational energy level diagram related to
fluorescence. http://www.resonancepub.com/spectrofluor.htm
(April 2, 2009).
- Spectrofluorimetry. http://www.resonancepub.com/spectrofluor.htm
(April 2, 2009).
- Moog, R.S., Spencer, J.N. & Farrell, J.J. (2004).
ChemActivity 22: Electronic spectra of atoms and molecules. Physical Chemistry: A Guided Inquiry:
Atoms, Molecules, and Spectroscopy, Houghton MIfflin Company,
New York, p. 209, 212, 213.
- Science Tech Entrepeneur,
July 2006 (2006).
http://www.techno-preneur.net/information-desk/sciencetech-magazine/2006/july06/Fluorescent_brighteners.pdf
(January 7, 2009).
- Image of
spectrophotometer (fluorimeter) from Wikipedia Commons. http://en.wikipedia.org/wiki/File:Spectrophotomer.JPG
(April 2, 2009).
- Molecular fluorescence spectroscopy. http://elchem.kaist.ac.kr/vt/chem-ed/spec/molec/mol-fluo.htm (April 3, 2009).
- Image of Jenway
62series fluorimeter. http://www.spectronic.co.uk/analytical-instruments/62seriesfluoro.htm
(April 3, 2009).
- PhotochemCAD Spectra by Category. http://omlc.ogi.edu/spectra/PhotochemCAD/html/index.html
(April 3, 2009).
|



 3.
Chemical Structure & Spectroscopy
3.
Chemical Structure & Spectroscopy Those optical brighteners that are particularly
effective whitening agents, or FWAs, work in a manner similar to bluing
to
increase the perception of whiteness; they are colorless (do not absorb
visible light) and absorb
ultraviolet light, "converting" it to emit blue light
(1). Figure 1 to the left shows an example of a common
substance that exhibits these properties: quinine in tonic water.
Those optical brighteners that are particularly
effective whitening agents, or FWAs, work in a manner similar to bluing
to
increase the perception of whiteness; they are colorless (do not absorb
visible light) and absorb
ultraviolet light, "converting" it to emit blue light
(1). Figure 1 to the left shows an example of a common
substance that exhibits these properties: quinine in tonic water.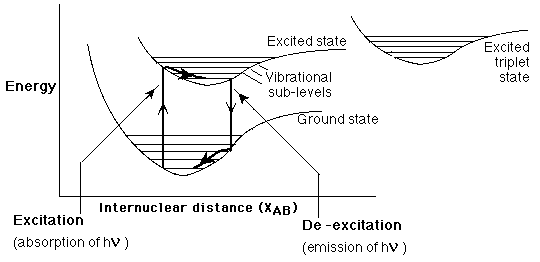 FWAs exhibit fluorescence as a result of
specific electronic and vibrational transitions, shown in Figure 3 to
the left:
FWAs exhibit fluorescence as a result of
specific electronic and vibrational transitions, shown in Figure 3 to
the left: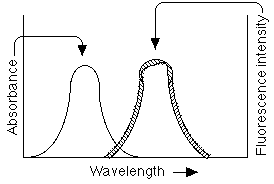
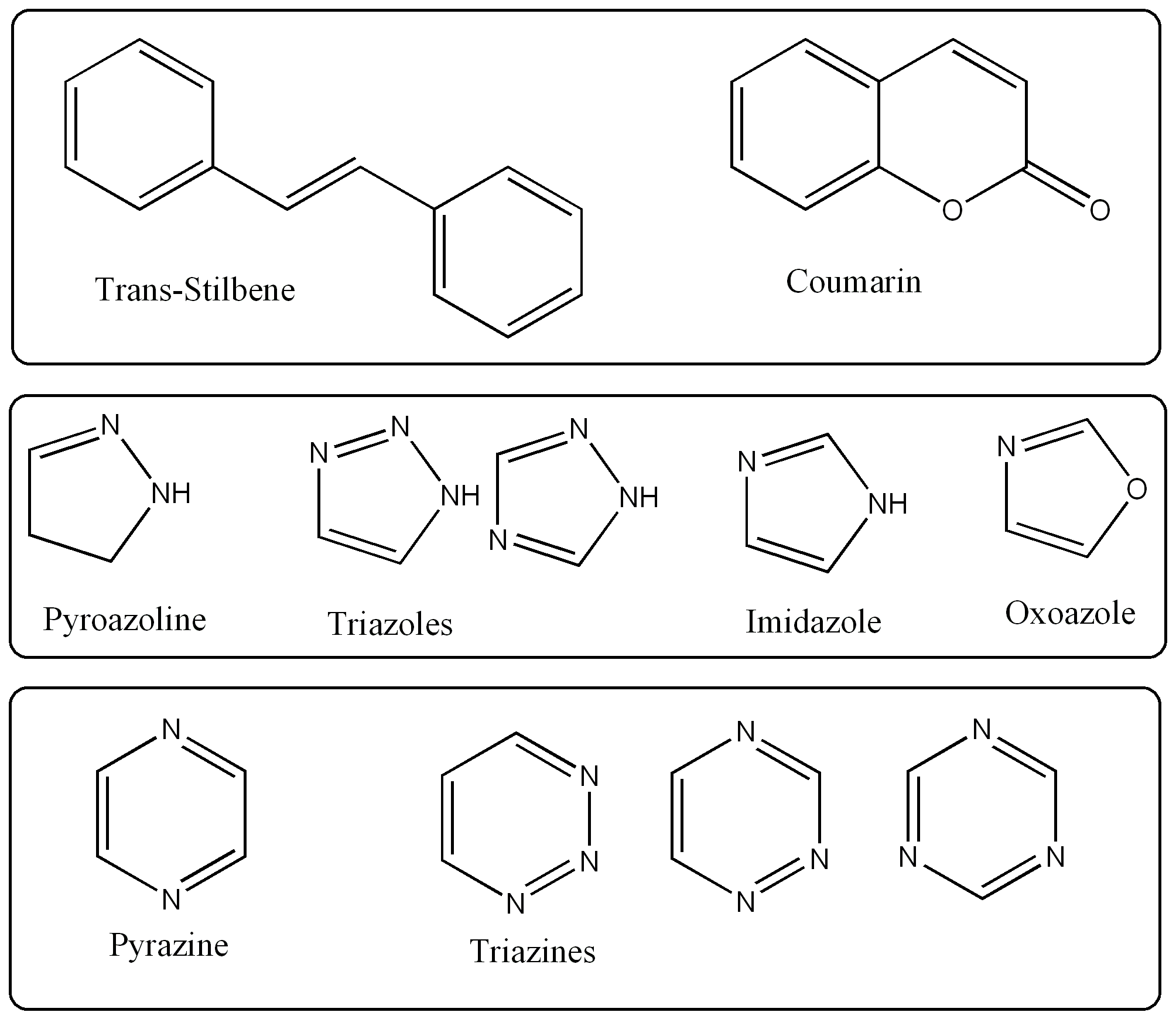 Most FWAs are
"derivatives of stilbene. . . biphenyl and five membered heterocyclics,
such as triazoles, oxoazoles or imidazoles. . . . six-membered
heterocyclics, such as coumarins, naphthalimide, pyrazine, or triazine
(7)." The
extensive pi-systems of these often heterocyclic aromatic compounds
are associated with the closely spaced electronic energy levels that
allow for energy transitions within the visible
range (e.g. n-->pi transitions). Table 1 to the right shows
some of base structures that
FWAs
are derived from.
Most FWAs are
"derivatives of stilbene. . . biphenyl and five membered heterocyclics,
such as triazoles, oxoazoles or imidazoles. . . . six-membered
heterocyclics, such as coumarins, naphthalimide, pyrazine, or triazine
(7)." The
extensive pi-systems of these often heterocyclic aromatic compounds
are associated with the closely spaced electronic energy levels that
allow for energy transitions within the visible
range (e.g. n-->pi transitions). Table 1 to the right shows
some of base structures that
FWAs
are derived from. By calibrating the wavelength of the initial light
used for excitation
useful to use a fluorimeter to measure the UV light
absorption associated with
the first electronic transition and the visible light emission
associated with the second electronic transition. A fluorimeter
is similar to a regular spectrometer except it must be outfitted so
that the light emitted by the sample cell must be carefulled filtered
(perhaps with slits or a diffraction grating) so that photodetector can
accurately assess absorption and emission of various wavelengths
(9). Thus, fluorimeters are high-end, sensitive spectrometers.
By calibrating the wavelength of the initial light
used for excitation
useful to use a fluorimeter to measure the UV light
absorption associated with
the first electronic transition and the visible light emission
associated with the second electronic transition. A fluorimeter
is similar to a regular spectrometer except it must be outfitted so
that the light emitted by the sample cell must be carefulled filtered
(perhaps with slits or a diffraction grating) so that photodetector can
accurately assess absorption and emission of various wavelengths
(9). Thus, fluorimeters are high-end, sensitive spectrometers.