|
|
|
|
|
Chemistry Class
page |
|
| Project home |
The Eye |
Retinal
And Rhodopsin |
Absorption
Spectra |
WebMo Job 992 |
Applications |
Links and
Refs |
University of Pennsylvania
Master of Chemistry Education Program
Chemistry 507: Molecular Spectroscopy
Do You See What I See?
Photoisomerization of Retinal
Jennifer Tareila
Spring 2004
| Spectroscopy:
The study of electromagnetic radiation (light) and its interactions
with matter.
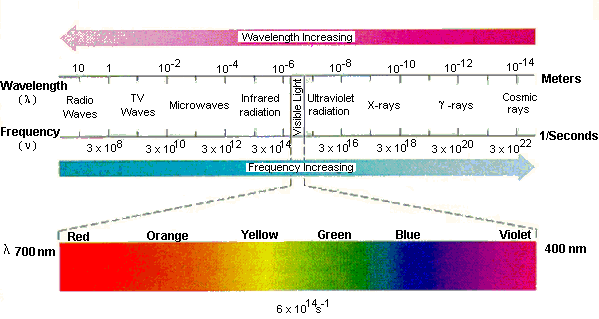
Interactions between light and matter can be used to learn about the chemical structure of the matter at hand, and can even tell if the matter is stationary or in motion. X-ray crystallography is used to determine the structure of molecules such as DNA, and spectral shift of light emanating from stars is used to determine if the stars are moving away from or toward us, giving us knowledge about the universe. However, the knowledge gained through spectroscopy are not just esoteric pieces of information that are not used by the average person, but pieces of information that apply to just about every person living in the developed world. We use polarized sunglasses, radios, televisions, remote controls, laser corrective surgery and fluorescent lights every day. There are photographs, glow in the dark stars, T-shirts that change color in the sun, and "black lights" that are used in forensic crime scene investigations. Medically, we use MRIs, CT scanners, and Xrays. These are but a few of the technologies the average person owes to spectral scientists that provided the information needed for scientists, doctors, and engineers to create these helpful and sometimes fun technologies. If you would like a more complete, yet still incomplete list, just search on Howstuffworks.com for electromagnetic spectrum or electromagenetic radiation; better yet, search the same string on Google for electromagenetic radiation (To find out more about electromagnetic radiation see this site from NASA.)
(image from http://chemed.chem.purdue.edu/genchem/topicreview/bp/ch6/atom_emr.html) Even more interesting is how spectra phenomena occurs in nature. Living things often produce their own light; jellyfish and plankton, as well as lightening bugs give off light. However, living things also depend on photochemical reactions- reactions of chemicals with light- to drive life processes. Plants absorb light and use the energy from that light to to produce sugars. Animals use light to see; the electromagnetic radiation is used to form a picture of the world around us. The brain interprets reactions of light with the photoactive molecules in the eyes. Humans see light in the visible range (approximately 400-700nm), while other animals can see in the infrared (IR, or heat) and ultraviolet (UV) ranges.
|
| When we see, the
are a multitude of events that take place at a molecular level. These
events take place at an extremely rapid pace. One of these events
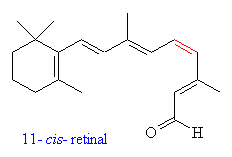
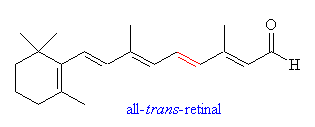
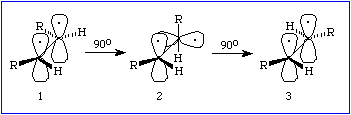
is the cis-trans isomerization of retinal, a visual pigment, by a photon
(the smallest piece of light). This process is referred to as a photochemical
reaction, or more specifically, a photoisomerization. The photoisomerization of molecules in the visible range is not unique to retinal. Bilirubins (18), azobenzene (37), tetrapyrole (16) and green flourescene protein in jellyfish (2) are all photoactive in this range. Many other biomolecules are active outside of the visible range, as are several organic molecules. One example would be butadiene, which will undergo a trans-cis imomerization at about 250nm(3). |