General Chemistry & Organic Chemistry I
Information & Experimental Chemistry
Dr. Bryan Roberts & Dr. William Dailey
Shape and energy (sterics)
Conformational
analysis of butane (Summer 2007):
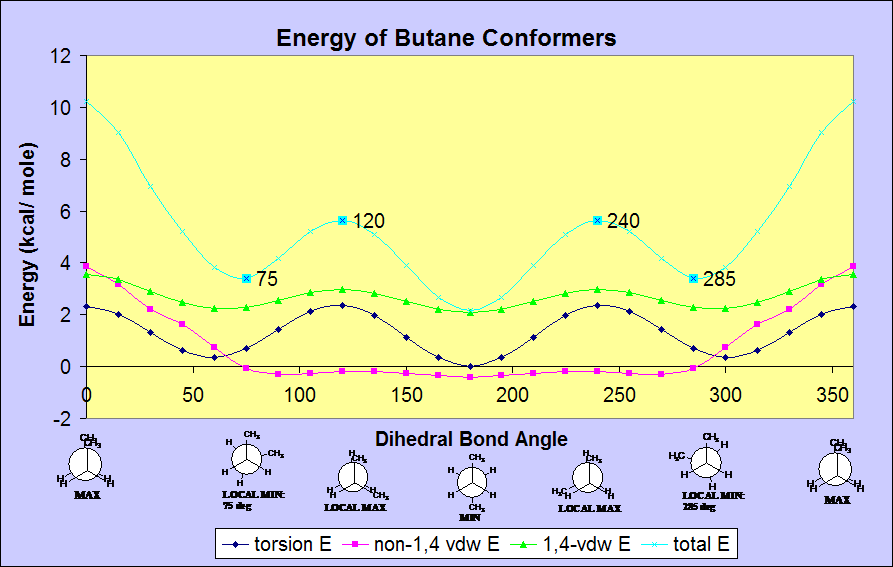

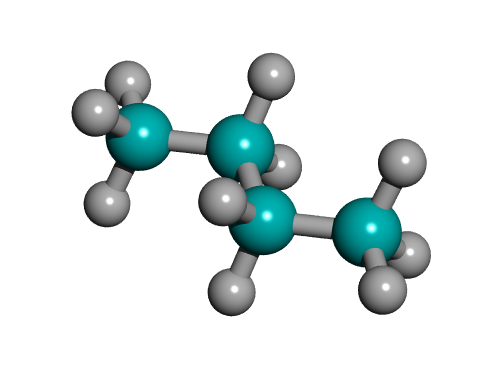
Conformational analysis of butane:
The
lowest energy, least sterically hindered conformation is the anti-
conformation (180 deg). The highest energy, most sterically
hindered conformation is the methyl-methyl eclipsed conformation (0 and
360 deg). As butane rotates around its C2-C3 bond, the energy of
the molecule changes to reflect the steric hindrance encountered in the
particular conformation.Organic Chemistry II
Dr. Bryan Roberts
Intermolecular attractions/ polymers (January 2008)
Macrogalleria
of polymers web
exploration: Polyethylene
(January 2008)

Intermolecular forces/ polymer PIM Question:
(January 2008)

(January 2008)
Intermolecular forces/ polymer PIM Question:
(January 2008)
Enduring Understanding (January 26, 2008):
V. THE PHYSICAL PROPERTIES OF A POLYMER ARE A FUNCTION OF POLYMER STRUCTURE.
a. Crosslinking increases the rigidity of a polymer.
b. Branching increases the intermolecular contact distances between polymer chains thus decreasing the dipole-dipole and/ or London dispersion attractions and making the polymer more flexible.
c. Polymer chains which interact through dipole-dipole and/ or London dispersion attractions tend to be strong but flexible and suitable for applications like fabrics, ropes, coatings, etc.
d. A highly regular polymer may pack so as to give regions of high crystallinity which make the polymer denser, stronger, and more rigid.
V. THE PHYSICAL PROPERTIES OF A POLYMER ARE A FUNCTION OF POLYMER STRUCTURE.
a. Crosslinking increases the rigidity of a polymer.
b. Branching increases the intermolecular contact distances between polymer chains thus decreasing the dipole-dipole and/ or London dispersion attractions and making the polymer more flexible.
c. Polymer chains which interact through dipole-dipole and/ or London dispersion attractions tend to be strong but flexible and suitable for applications like fabrics, ropes, coatings, etc.
d. A highly regular polymer may pack so as to give regions of high crystallinity which make the polymer denser, stronger, and more rigid.
HDPE can be used to make bulletproof vests, while LDPE can be used to make lightweight (relatively flimsy) disposable plastic grocery bags.
HDPE:
Linear polyethylene has a shape conducive to close packing of polyethylene fibers and extensive London dispersion interactions between each strand. This produces a tightly bound, high density product that can be used for applications that require rigidity and strength.
LDPE:
Highly branched polyethylene, however, has a shape that resists close packing, and thus, has low levels of London dispersion forces. This produces a light, low density product that is ideal for applications that do not require strength, but do require flexibility.
Biochemistry & Molecular Biology
Dr. Stacy Gelhaus
Enzyme substrate specificity/ allosteric inhibition (hemoglobin)
Enzyme/ substrate shape (July 2009 .ppt):
 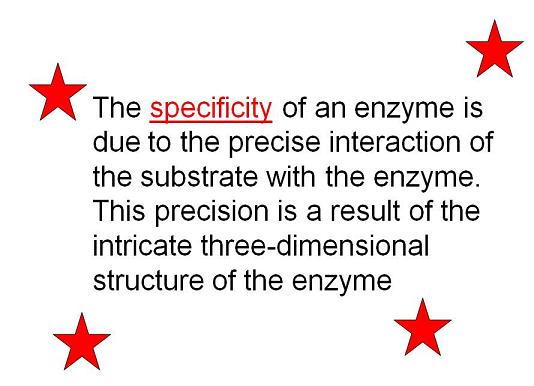 |
Slide 1-3:
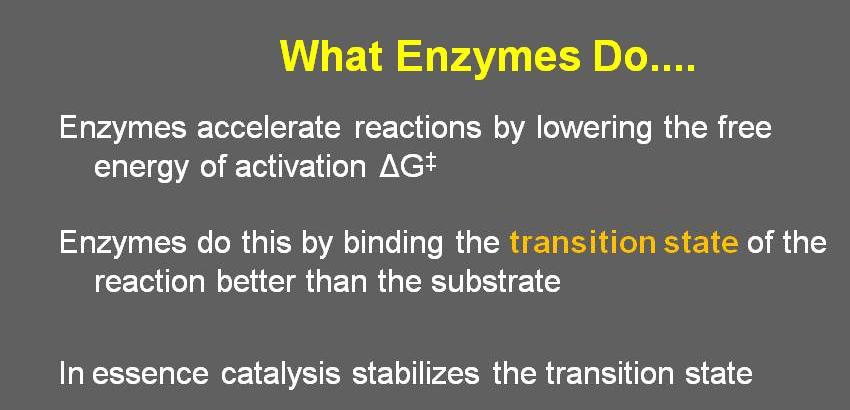
The active site of an enzyme is designed to specifically bind to a particular substrate (with a particular shape and polarity) and catalyze a specific reaction by stabilizing the transition state. As mentioned in Slide 2 to the left, "this precision is a result of the intricate three-dimensional structure [i.e. shape and polarity] of the enzyme."
Slide 4:
Because shape plays such an important role in enzyme-substrate interactions, transition state analogs (which are bound by the enzyme, but cannot be acted upon) can effectively inhibit an enzyme.
ALLOSTERIC
REGULATION:
A brief discussion of oxyhemoglobin (oxygenated tetramer in blood), deoxyhemoglobin (deoxygenated tetramer in blood), myoglobin (monomer in body tissues), and fetal hemoglobin.
Furthermore,
certain enzymes and proteins (such as hemoglobin) can be regulated allosterically--meaning that their function
can be altered by a change in shape (i.e. conformation). A brief discussion of oxyhemoglobin (oxygenated tetramer in blood), deoxyhemoglobin (deoxygenated tetramer in blood), myoglobin (monomer in body tissues), and fetal hemoglobin.
- When oxygen binds
hemoglobin, the protein changes shape, decreasing the size of the
"hole" at the center of the protein.
- BPG's shape and polarity allow it to bind hemoglobin to stabilize deoxyhemoglobin, so that hemoglobin has a lower affinity for oxygen than myoglobin (this is good--tissue cells can get the oxygen from hemoglobin)
- two amino acid in
fetal hemoglobin are different (serine for histadine-143 in the beta
units), decreasing BPG affinity, this gives fetal hemoglobin a higher
affinity for oxygen (this is good--the fetus can get the oxygen from
mom's blood)
DENATURING AN ENZYME
Enzymes that lose
their shape because of pH changes, heat, or disulfide bond disruption
lose their function until they refold into the active conformation.
Environmental Chemistry
Dr. Mark Hermanson & Leslie Anderson
Shape and biotoxicity
Pesticides:
DDT's effectiveness as a pesticide and as a
hormone mimic (August 4, 2008 .ppt)
Carcinogenic Toxins: Dioxins, Furans, and
planar PCBs (August 11, 2008
.ppt)
DDT is a nonplanar compound used as a pesticide. Its use is now banned in the US because of its persistence (it is very resistant to oxidative, hydrolytic, or photolytic degradation), its tendency to bioaccumulate (Kow=100,000), and its interaction with the human estrogen receptor (i.e. action as a hormone mimic). While DDT's ability to bioaccumulate has much to do with its lipophilic phenyl rings,
- DDT's
nonplanar
shape (due to the sp3 hybridization of its central
carbon) and the steric repulsions of its chlorine substituents are responsible for its potency as an
insecticide, and
- the shape of its phenyl rings makes it a good mimic for estrogen, which also contains a phenyl ring.
******************************
Other toxins, such as dioxins, furans, and other polyaromatic hydrocarbons can be carcinogenic because of their planar shape. In these compounds, the addition of large halogen substituents can reduce the carcinogenicity of the compounds by forcing the compound (via steric repulsions) into less toxic nonplanar conformations.
Inorganic Chemistry
Dr. Donald Berry
August 2008 notes and problem set:
Crystal Field Theory & Strong vs. Weak ligands
Crystal Field Theory & Strong vs. Weak ligands
|
Activity:
(click on image for larger view) |
The magnitude of the CFSE is affected by:
- shape of the
coordination environment (octahedral, tetrahedral, or square planar (D4h))
- ligand "strength", those ligands with a more intense negative charge will induce greater splitting.
- the metal identity (i.e. size, charge, number of electrons)
Square Planar coordination environments split further (imagine the effect of removing 2 ligands along the z axis), so that the x2-y2 orbital and z2 orbital split (in this case x2-y2 is the higher energy one) and the three xy, yz, and xz orbitals split (one over two--in this case xy orbital is higher energy than the yz and xz orbitals).
Tetrahedral coordination environments have a characteristic three over two splitting of d orbitals because the xy, xz, or yz orbitals experience greater overlap with ligands than the x2-y2 and z2 orbitals.
Molecular Spectroscopy
Dr. Susan Phillips & Dusty Carroll
ChemActivity
21 HW
Answers: IR Spectroscopy, Questions #8-10 (January 7, 2009)
(click on image for larger view)

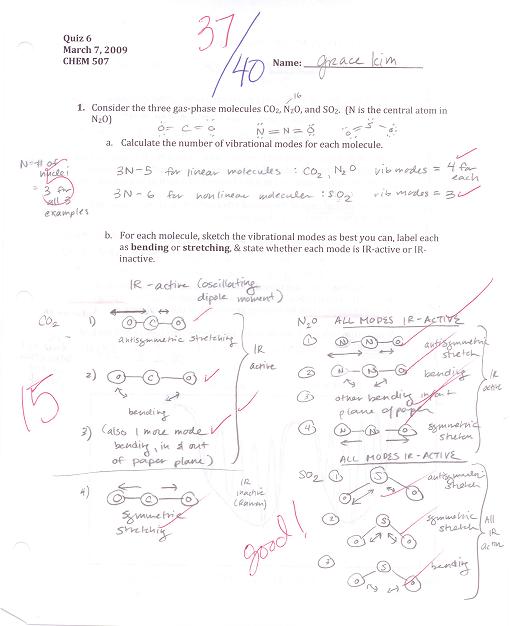
Quiz #6 (March 7, 2009)
(click on image for larger view)
(click on image for larger view)
Quiz #6 (March 7, 2009)
(click on image for larger view)
Molecules have various vibrational modes in which their bonds stretch or bend. Any vibrational mode that causes a change in the overall dipole moment of the molecule will absorb IR radiation, and can be detected by IR spectroscopy. Those vibrational modes that do not change the overall dipole moment of the molecule can often be detected by Raman spectroscopy.
CO2 (symmetric, linear molecule) vs. H2O (bent molecule) and NH3 (trigonal pyrimidal)
CO2 has 4 vibrational modes. Because of the symmetric shape of the molecule, the symmetric stretch is IR inactive because the dipole moment of the molecule does not change. The other 3 modes (2 assymetric bending--in/out of the x-y plane, in/out of the x-z plane; 1 assymetric stretching) are all IR active. (See
http://www.chem.purdue.edu/gchelp/vibs/co2.html, which requires Chime).
In comparison, all the vibrational modes of H2O and NH3, which are not symmetric, can be detected with IR spectroscopy.
In conclusion, through continually having to revisit the connection of shape to various properties throughout the MCE coursework, I have further realized the importance of shape. The natural shape of a molecule is that which confers the most favorable (lowest) energy profile--VSEPR explains how molecules move their bonds and lone pair electrons as far away from each other to minimize e- to e- repulsions. In the case of coordination compounds, the introduction of ligands yields electron configurations that are characteristic to the shape of the coordination environment.
Shape, whether molecular geometry (which is a function of connectivity and atomic composition) or conformation (which is a function of folding or rotation around sigma bonds), directly impacts the energy profile and properties of the substance. Biological interactions, such as those between enzymes and subtrates or between signalling molecules and cell receptors, are often mediated by the "fit" (which is a combination of shape and polar interactions) between two molecules. In polymers and plastics, macroscopic properties are often determined by the intermolecular interactions at the submicroscopic level, which are strongly influenced by molecular shape and how closely molecules can pack. In spectroscopy, the symmetry and/ or shape of a molecule can impact the magnitude (and therefore) type of energy that is absorbed. The symmetry of a compound affects the detection of its vibrational modes in IR spectroscopy (since only vibrational modes that change the molecular dipole moment absorb IR energy). For coordination compounds and complex ions, the shape of the coordination compounds is highly related to the energy levels of the transition metal's d orbital (those with greater overlap with ligands are promoted to higher energies), this affects the absorption of energy and the number of unpaired electrons (whether the complex is dia- or paramagnetic).