| EVIDENCE #1: Integrating an Understanding of Coulomb's Law Into the Intermolecular Force UNIT |
|||
|
THE CONTENT
|
|||
Baseline evidence:
|
Later
evidence:
|
||
|
I had NOT taught Coulomb's Law as the basis for intermolecular attractions or periodic properties related to shielding before taking Chem507. Old worksheets on intermolecular forces
|
From content-integration
project in Chem503:
(click on image to see larger view of highlighted view) Intermolecular Force Reading Assignment
p. 2, Questions #6-12 (click here for full .pdf) (click on image to see larger view of selection section) Coulomb's Law POGIL & HW (click on image for full .pdf) Intermolecular Forces POGIL (click on image for full .pdf) |
||
|
REFLECTION ON GROWTH
Comparison of the
baseline and later evidence shows a shift from having students learn
only what an intermolecular force is and the properties it is related
with toward having students also connect this understanding to
Coulomb's law of electrostatic attraction (a law of physics). I
would not have integrated these concepts and pushed my students toward
a deeper understanding (one based on physical laws) had I not
encountered Dr. Robert's Coulomb's Law POGIL in Chem501. Future
growth (in the coming years) will consist of trying to integrate this
law into other units where it applies (e.g. shielding in the periodic
properties unit).
|
|||
| EVIDENCE #2: Integrating Lessons on Color Perception and Fluorescence Into the Quantum Theory UNIT |
|||
THE CONTENT
|
|||
| Baseline evidence: OLD LESSON PLAN: (Electromagnetic radiation and quantum theory) 2008-2009 school year |
Later evidence: NEW LESSON PLAN: (Color vision and fluorescence) planned for 2009-2010 school year JANUARY 2010 |
||
I had NOT taught color perception or fluorescence in concert with my electromagnetic radiation and quantum theory before taking Chem507. Day 1-2: Electromagnetic radiation/ intro to quantum theory with atomic emission spectra lesson Note sheet
(click on image for full .pdf) -Atomic
emission spectra applet
-Students use spectroscopes to view various light sources (mini-lab activity); results are discussed in class; reviewed with notes Day 3: Bohr model lesson -Bohr model applet and website with wavelengths of emission, students asked to do calculations of energy transitions corresponding to visible light Applet
(click to link to applet site) Day 4: Flame test lab -student observe characteristic colors of chloride salts of Li, Sr, Ba, K, Na, and Cu, and although the colors observed are a composite of various emitted wavelengths, students choose a main "representative" wavelength for the color seen and calculate the frequency and energy associated with the emission of that color light Day 5: QUIZ Day 6-7: Electron configurations/ atomic orbital diagram lessons -lecture/ several worksheets (some representative worksheets shown) E- configuration
worksheet 1
(click on image for full .pdf) E- configuration Worksheet 2 (click on image for full .pdf) Day 8-10: -QUIZ -Review -TEST |
Previous Days: -electromagnetic radiation lesson -intro to quantum theory with atomic emission spectra lesson Day 1: POGIL PhET applet
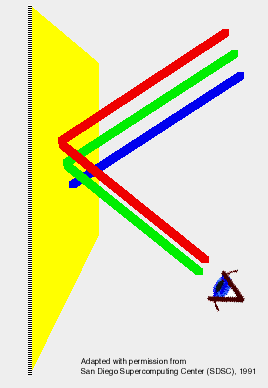
activity on color vision
(click on image for full .pdf) The color applet activity that describes how various wavelength (i.e. color) lights produce a particular perception of color (e.g. a combination of green and red light creates the perception of yellow colored light) would help students understand how the different bands of light in an element's emission spectrum produce the perception of a particular color (e.g. the pinkish glow of an argon spectral tube even though its emission spectrum shows blue, green, and red bands). Furthermore, I thought it would be an interesting foundation concept to learn, especially for students interested in stage lighting and photography. Day 2: -discussion/ review of POGIL PhET applet activity -small QUIZ on electromagnetic radiation/ color perception -(some lecture/ old lessons on quantum theory) Day 3-6: -continue old lessons on quantum theory (electron configurations, atomic orbital diagrams) -do flame test lab -small QUIZ on quantum theory -some lecture on fluorescence Day 7: Fluorescence worksheet
(click on image for full .pdf) Day 8-9: Fluorescent brightening agents in laundry
detergent LAB
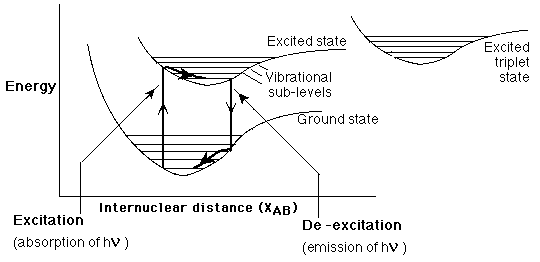
(click on image for full .pdf) Day 10: TEST Beyond the use of fluorescent light bulbs, most students are unaware of how pervasive fluorescence-based applications are. The ability to absorb higher energy, invisible UV light and emit lower energy visible light has allowed for the creation of "brighter" pigments and colors that can also appear "whiter" if the proper wavelength light is emitted (going back to the first, color perception lesson). I thought this lesson would connect quantum theory with something more tangible in the everyday routine of an average student. |
||